| |
II./2.5.: Teratoma in other localizations: gonadal teratomas and other germ cell tumors, demonstrated on the example of testicular cancer
II./2.5.1.: Germ cell tumors, in general
According to the histopathologic classification there are two main groups of germ cell tumors:
|
 |
-
1. Tumors containing only one histological type, like: seminoma, spermatocytic seminoma, embryonal carcinoma, yolk sac tumor (endodermal sinus tumor), polyembryoma, choriocarcinoma, teratoma; and
-
2. Tumors containing more than one histological type (composit testicular cancers): tumors constituting of any mixture of the previously listed tumor types. Although, germ cell tumors are rare in proportion to all of the tumors, in young age groups (during the first 3 years and between the ages of 15 and 45 years) they are rather frequent.
During embryogenesis, germ cells originating from the wall of the yolk sac, migrate along the midline to the primordial gonadal ridges. If an error occurs in the program of the migration, germ cells may stuck anywhere along their way. This may explain why germ cell tumors occur not only in the gonades (testicules and ovaries), but at different levels of the midline, like in the retroperitoneum, in the mediastinum, moreover, in the area of the corpus pineale. Taking into consideration the recent, remarkably effective treatment results, this group of tumors deserve special attention. Apart from the precise histological diagnosis, it is also important that these tumors express serologically detectable proteins (so called tumor markers), with the help of which, both tumor progression and therapeutic efficacy can be followed.
|
 |
The prevalence of germ cell tumors is 2-3/100 000. However, if we focus on the age group of 20-30 years, prevalence is cca. 5.8-7.2/100 000. In this age group, germ cell tumors are considered as the most frequent solid tumors, and unfortunately these tumors are also the most frequent causes of death. In the US, for example 90% of the germ cell tumors occur before the age of 45. Taking into consideration all of the tumors, however, a triple peak can be observed on the distribution curve: during infancy, young adulthood and after the age of 60 years.
II./2.5.1.1.: Pathophysiology
The cause of germ cell tumors is unknown, but causative effect of some factors can already be assumed. The role of genetic factors seems obvious, since prevalence is increased in siblings, in monozygotic twins, and among family members. If the tumor occurs in one side, there is high risk for the occurrence on the other side. In case of germ cell tumors, surgical intervention that needs to be done, is complete castration. Tumors often develop in dysplastic (incompletely, or abnormally developed) gonades.
|
 |
It is a well known fact by know, that the risk for the development of germ cell tumors is 33 times higher in descended testicules compared with undescended testicules, most probably due to the higher temperature. In the anamnesis, mumps affecting the testicules, as well as orchitis individually occurring, or co-occurring with another disease, are frequently reported. Several patients report about antecedent trauma, however, in these cases it is more likely that the trauma only draws the attention to the tumor that has already been there. According to certain data, chronic LSD abusers have higher risk for germinal tumors. It can refer to the role of endocrin factors that one of the prevalence peaks is in young adulthood, since this co-occurs with the peak of change in sexual hormones. Experimentally, germ cell tumors may be induced by zinc and copper injections. In summary: cryptorchism, mumps and other orchitises, as well as genetic factors can be taken as the most important risk factors of testicular cancers.
II./2.5.1.2.: Symptoms
Gonadal germ cell tumors do not have specific early symptoms, only in case of testicular cancers, the enlargement of the organ can be palpable, and possibly it becomes more sensitivite than usual. Often the patient or the doctor examining the patient due to another complaint, or even the sexual partner notices the toughening of the testicles, or the tumescence, that developed within the testicular parenchyma, and became gradually palpable from outside. Gynecomastia occurs in cca. 2-10% of the cases, thus in case of gynecomastia, testicular cancer always has to be considered. In cca. 10% of the cases, acute symptoms mimicing epididymitis, torsion, or testicular infarction occur, due to the haemorrhage, or infarction of the tumor tissue. Unfortunately, in approximately 25% of the cases, at the time of the discovery of the tumor, there are already developed, generalized metastases. Extremely rarely, infertility might be one of the initial symptoms.
II./2.5.1.3.: Diagnosis
|
 |
Complete physical examination is necessary. One or both of the testicules may be enlarged, and be flexibly dense, rubber-like with palpation. Mostly one, but sometimes multiple nodules can be palpated. The shape of the testicule is often intact. The tumor often demarcates from the epididymis, and does not clinch to the skin. Currently, in case of suspecting testicular carcinoma, the examination of two tumormarkers are necessary for the diagnosis, staging, and monitoring: human beta-choriogonadotropin (β -HCG) and the α-foetoprotein (AFP). The elevated level of β-HCG suggests the presence of choriocarcinoma, but at least syntitiotrophoblasts in the primary tumor, or in the metastases. It is important to note that elevated level of β-HCG may occur independent of germ cell tumors in 8% of the cases, thus it can be false-positive, however extreme increase of its level is practically the marker of the tumor proliferation.
Evaluating the elevated level of AFP is more complex. In human embryo, AFP is produced by the yolk sac, and later by the liver. Thus, in adults, the elevated AFP level is physiological only during pregnancy. In case of testicular cancer, the increase of the level of AFP frequently co-occurs with yolk sac elements, however, this may occur also in embryonal carcinoma and undifferentiated teratoma either. Elevated level of either tumormarkers is present, in approximately 70% of the cases. Half-life of β-HCG is 24 hours, of AFP is 5 days. If either markers stay elevated one week after the removal of the tumor, it suggests that a metastasis has already developed somewhere. Regarding clinical classification, the most important issue is whether the tumor is localized only in the parenchyma, or the adnexes are also infiltrated; regarding the remote metastases, it is important whether metastases are only under, or beyond the diaphragm.
|
 |
In case of suspecting a testicular carcinoma, biopsy is contraindicated, because based on evidences, it increases the risk for local recurrence; this is by the way quite rare. The removal of the testicules (orchiectomy) is the first step to be chosen, that has to be followed by detailed histological analysis of the tumor. It is important to decide whether the tumor is constituted of one component, or it is a complex tumor. Taking into consideration the fact that without treatment, germ cell tumors metastatize in high percent of the cases – and this causes the death of the patient eventually – in case of systemic expansion, complex tretament (surgical, pahramacological and irradiation) is necessary. The factors determining the treatment to be applied:
-
a.) what is the histology of the primary tumor,
-
b.) are there clinically justified metastases,
-
c.) whether after orchiectomy, tumormarkers mentioned above persist.
Currently, high percent of the testicular carcinoma cases are treated successfully.
II./2.5.1.4.: Histogenesis and intratubular (in situ) processes
Because of the different treatment protocols, the differentiation of
-
- clear seminomas
-
- non-seminomatous germ cell tumors, and
-
- mixed types of these, has special clinical significance.
The morphological diagnosis of clear seminomas usually is not a problem, however, technically it is almost impossible to analyze the whole sample, in case of large testicular carcinomas, and thus the evaluation whether the tumor contains non-seminomatous elements too. Of course, the measurment of serum AFP- and β-HCG levels is a great assistance, because their significant elevation always suggest the possibility of non-seminomatous elements being present. Recent studies support the notion that non-seminomatous germ cell tumors originate from seminomas, mainly due to further genetic lesions.
Intratubular germ cell neoplasias (Micrographs 4A-B) are mainly tetraploid, clear seminomas are tetraploid or hypotetraploid, while non-seminomatous tumors usually become aneuplid due to DNA loss. The DNA determination has practical significance in reinforcing the diagnosis in case of clear seminomas, when detailed evaluation of the sample suggest clear seminoma, immune-histochemical examinations do not find non-seminomatous elements either, and there is no elevated AFP or β-HCG level. This means that the tumor can be conservatively treated, because seminomas in clinical stage I need only surgical intervention, in which the removal of the tumor and the testicule are necessary but not the removal of the retroperitoneal lymphnodes. Furthermore, chemotherapy can be delayed, and if there are no metastases it is omissible.
|

Look into the figures and analyze what you see!
|
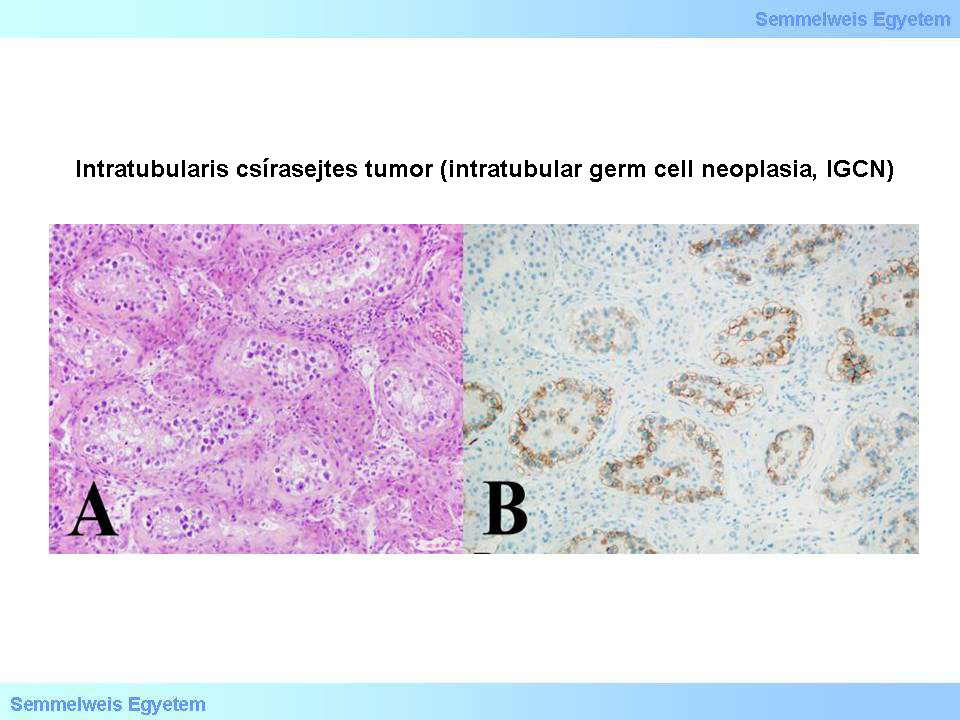
Micrographs 4A-B: Intratubular germ cell neoplasia (IGCN). A (HE; 200x): Around the edge of testicular tubules with fibrotic wall, showing no spermatogenesis, in continuous or dashed lines, often grouped on each other, atypical tumor cells are positioned which remind to germ cells, but are bigger, have wide and clear cytoskeleton, and enlarged nuclei with prominent nucleoli.These cells obviously originate from between the rest of the germinal epithelium and the basal membrane of the tubules, or they crawl in under the germinal epithelium. B (CD117; 200x): Atypical cells – similar to the tumor cells of invasive seminoma – show c-kit (CD117)positivity. This reaction directly shows the peripheral position of the tumor cells, within the tubule. In the upper left corner of the picture, within the intact tubules, there is no positive signaling. (From the archive of the Semmelweis University, 2nd Department of Pathology–collection of Ildikó Szirtes)
|
II./2.5.2.: Non-seminomatous germ cell tumors
II./2.5.2.1.: Teratoma
|
 |
Testicular teratomas are (similar to teratomas in other locations) complex tumors, in which there are elements from not only one germ layer. Clear teratoma gives 2-3% of germ cell tumors in adults, and approximately 40% of testicular tumors in infants and children. In cca 45-50% of composit testicular carcinomas, teratoma part can be found. AFP level can be slightly elevated. These tumors are middle size, greyish-white, typically cystic, and may contain bone, or cartilage, as well as sometimes tooth (molar type) with chewing surface and root, embedded in the cyst wall. In the epithelial cells of the cysts there can be keratohyalin or mucin. Contrary to the ovaries, dermoid cyst in the testicules is extremely rare.
Histologically, teratoma may show quite a various picture, it can contain both embryonic and adult tissues. Three types of teratomas can be differentiated: mature teratoma, unmature teratoma and teratoma with malignant transformation. (Earlier, mature teratoma [teratoma maturum] was called teratoma adultum, unmature [teratoma immaturum] was called teratoma embryonale; incorrectly, because their appearance does not depend on the age of the patient.) In babies and children, mature teratoma is the most common, in these cases well—differentiated structures can be seen (e.g. cartilage, smooth muscle, glandules, alveolar epithelium, gastro-intestinal structures, squamous epithelium, transitional epithelium, neuronal tissue). In adults, purely mature teratomas are rare, thus, it is always essential to carefully look for unmature or malignant elements.
Unmature teratomas mainly include primitive ectodermal structures, primitive chondroid tissue, mesenchymal tissue particules. AFP can be detected in both mature and unmature teratomas. Teratomas carrying malignant transformation include malignant degenerations of the constituting tissue parts (e.g. rhabdomyosarcoma, neuroblastoma, epithelial carcinoma, mucinous adenocarcinoma etc.), occurring also in other locations. Teratomas in babies have generally better prognosis compared with those in adults. Teratomas carrying malignant transformation show the worse prognosis.
II./2.5.2.2.: Choriocarcinoma
|
 |
Clear choriocarcinoma is one of the most malignant germ cell tumors, it is rare in testicules. The tumor is usually small, almost always haemorrhages and necroses can be observed on its cut-surface, surrounded by a thin layer of normal tissue. It is typical, that first symptoms are caused by the metastases (so called occult carcinoma), in young adults, around the age of 20 years. This can make the diagnosis very difficult in certain cases, since if it is not suspected, the small primary tumor would only be revealed during the autopsy. Serum β-HCG level is quite high, and gynecomastia is also observable in most of the cases. For the histopathological diagnosis of choriocarcinoma, two cell types are necessary: malignant versions of cytotrophoblasts and syntitiotrophoblasts. Malignant cytotrophoblasts have only one, large, hyperchrome nucleus.
Tumor cells form bundles, often they create villous structures. At the edges of these structures can tumorous, multi-nuclei syntitiotrophoblasts be found (Micrograph 5). There is no specific immunologic marker for cytotrophoblasts, while syntitiotrophoblasts are β-HCG positive. Nonetheless, clear choriocarcinoma is extremely rare, while either choriocarcinoma or only syntitiotrophoblasts can be found in small areas of other types of germ cell tumors. The mortality of gonadal choriocarcinomas are high, despite the intensive treatment, both in males and females. In females, however, apart from gonadal choriocarcinoma, so called gestational choriocarcinoma can also occur, related to pathological pregnancy. The latter has good prognosis, because chemotherapy (e.g. platina based substances /cisplatin/, or metothrexate) showed to be very effective in its treatment, after which complete recovery and successfull pregnancy are both possible.
|

Look into the picture!
|
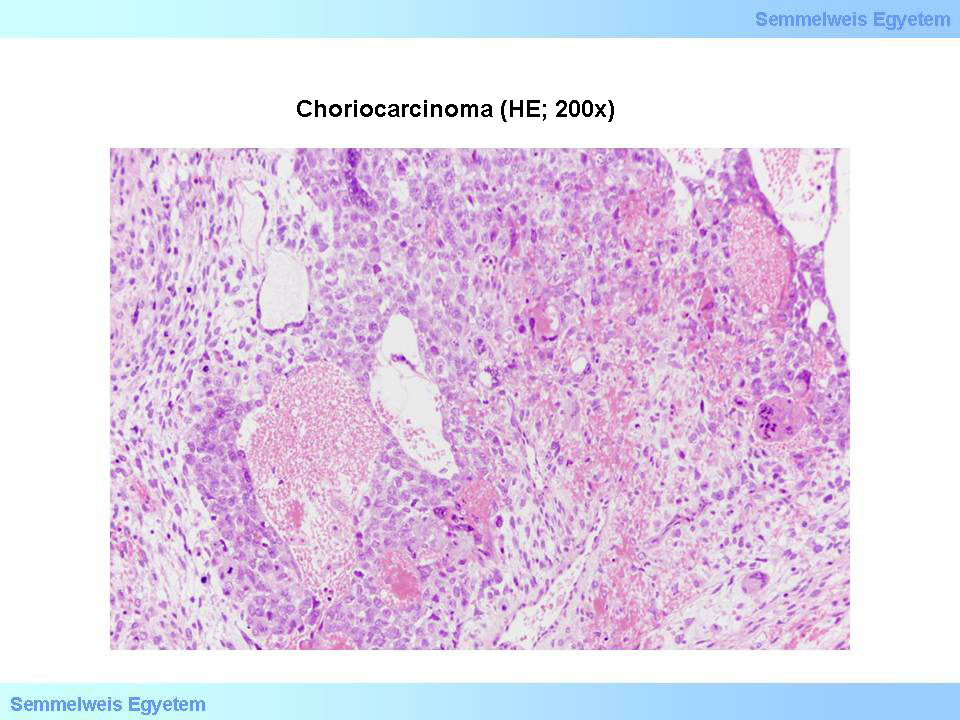
Micrograph 5: Choriocarcinoma (HE; 200x). Tumor tissue with haemorrhages and necrosis, constituted of malignant cyto-, and multinuclei syntitiotrophoblasts. (From the archive of the Semmelweis University, 2nd Department of Pathology–collection of Ildikó Szirtes)
|
II./2.5.2.3.: Yolk sac tumor (infantile embryonal carcinoma, endodermal sinus tumor, orchioblastoma)
The tumor reminds to the embryonal yolk sac (endodermal sinus). This is the most frequently occurring testicular cancer in infancy and childhood. AFP level is typically high. The cut-surface of the enlarged testicule is drab, mucinous-like, granular. The tumor shows reticular pattern, histopathologically: the tumor cells are cuboid or flat, they form tubular structures, or opening to each other. The cuff-like tumor cell bundles, organized around small blood vessels are the so called Schiller-Duval-bodies (Micrograph 6). As a result of treatment, 5 year survival of babies and children is over 80% of the cases.
|

Look into the picture!
|

Micrograph 6: Yolk-sac tumor (HE; 100x). Within the tumor tissue reminding to the endodermal sinus, tumor cells typically pile up around small blood vessels (so called Schiller-Duval-bodies). (From the archive of the Semmelweis University, 2nd Department of Pathology–collection of Ildikó Szirtes)
|
II./2.5.2.4.: Polyembryoma
This extremely rare tumor constitutes of reminding to embryonal structures. Its histological picture reminds also to the histology of a 2 weeks old embryo. Endodermal type tubular structures, as well as syntitiotrophoblasts can also be present.
II./2.5.2.5.: Embryonal carcinoma
Clear embryonal carcinoma represents only 3% of germ cell tumors, but such regions can be found in 42% of testicular carcinomas. In clear embryonal carcinoma β-HCG level is not elevated, however, the level of AFP may be. It deformates the shape of the testicule more than seminomas do, because it frequently infiltrates the sheath and the epididymis too. The cut-surface is mainly soft, greyish, or greyish-red, with necrotic parts and haemorrhages. This tumor is rarely cystic.
|
 |
Histologically it is characterised by tumor cells that are only sometimes cohesive, other times they form more or less differentiated follicule-like structures (Micrograph 7). Cells are similar to seminoma cells with regard to nuclei and nucleoli, but their cytoskeleton is not that clear. Mitotic activity is high. The stroma does not show lymphoid infiltration. This tumor is less sensitive to radiation compared to seminoma, however, chemotherapy is effective, it reduces mortality significantly. Earlier, in case of embryonal carcinoma retroperitoneal lymph nodes, and lymph-node chain were removed on a regular basis for staging purposes, which method is not anymore considered necessary. Introducing chemotherapy, the life expactancy of patients with embryonal carcinoma improved substantially. Specifically, in the ’70-s around 75% of the patients died in 5 years, while from the ’90-s, cases without metastasis have 80% survival rate over 5 years, but even those with metastasis approach the 70% survival rate.
|

Look into the picture!
|
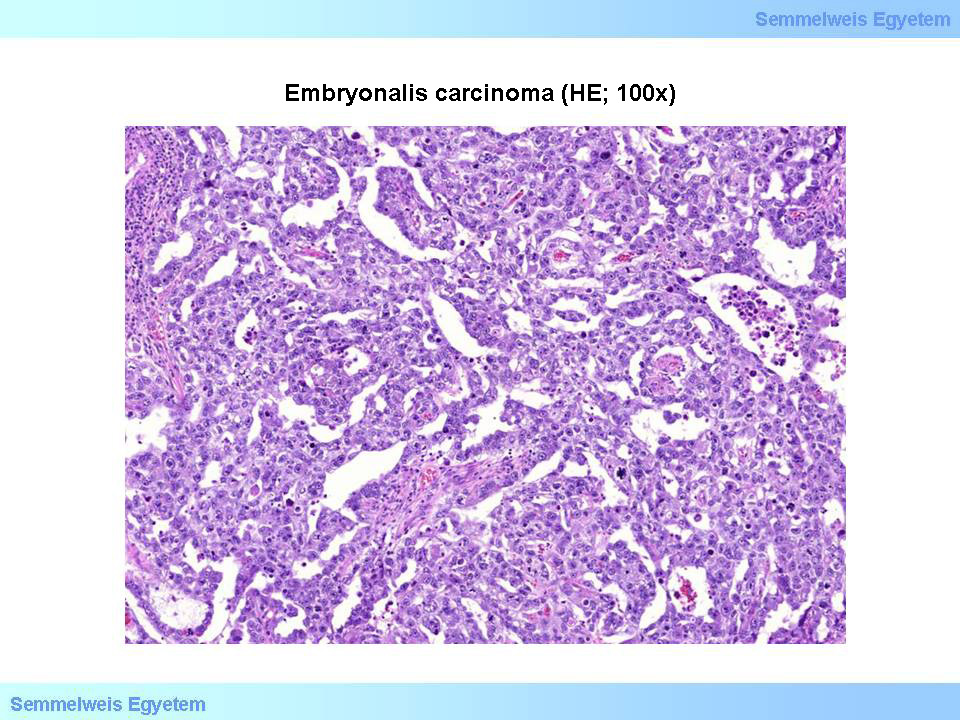
Micrograph 7: Embryonal carcinoma (HE; 100x). Tumor cells, reminding to germ cells, with polymorph, prominent nucleoli, create abortive glandular structures. (From the archive of the Semmelweis University, 2nd Department of Pathology–collection of Ildikó Szirtes)
|
II./2.5.3.: Seminoma
|
 |
Compared with the other germ cell tumors, seminoma typically occurs in later years (its peak of frequency is at the years of the thirties), and is relatively less malignant. It is more frequent in cryptorch testicules, than any other germ cell tumor. Thirtyfive-fourty percent of all germ cell tumors, and 72% of monocomponent tumors are seminomas. In clear seminoma, neither the AFP, nor the β-HCG level is elevated. Its cut-surface is mainly white, medullar, but it can be yellowish, or tawny. Sometimes it is completely homogenous, other times it is lobular. Large tumors usually occupy the whole testicule, while small ones are demarcated within the testicular parenchyma, but they do not have sheath. Necrosis occurs, but haemorrhage is rare, there are practically no cystic areas.
Histologically, monomorphic seminomas can easily be recognized based on the monomorphic cellular picture. Cells are mainly middle sized, round, or cubic, rather uniform with expressed cellular borders. The cytoplasm contains glicogen, and thus, it is clear. The nucleus is centered, large, round, nucleoli – dominantly one or two – are slightly eosinophilic, well observable. Mitotic activity is not salient, but sometimes it can be increased; these cases are considered more agressive seminomas. Tumor cells do not really form special structures, sometimes they form bundles, or columns. It is very typical that in between the cells there is a fine stroma creating a connective tissue like hedge-network, in which there is always lymphotic infiltration to various extent (Micrograph 8A). When sometimes the stroma is expressed, the tumor is lobular, that can be observable even macroscopically. PLAP-reaction is positive in 90% of the cases, further markers of the tumor are c-kit (CD117), and OCT3/4 (Micrograph 8B).
|

Look into the picture!
|
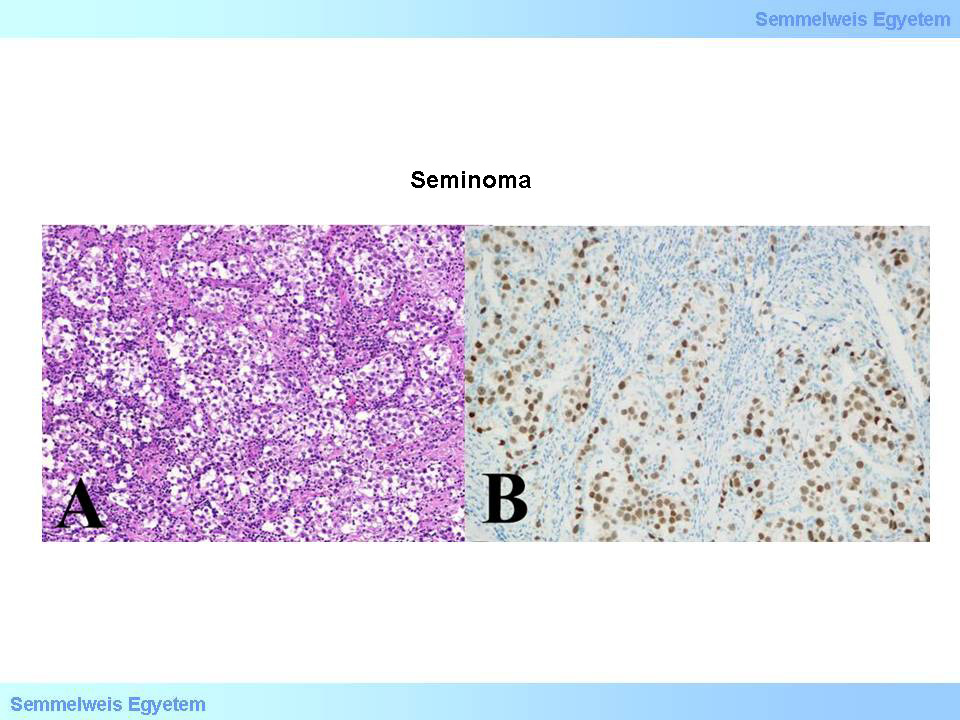
Micrographs 8A-B: Seminoma. A (HE; 200x): Tumor tissue, constituted of tumor cells greatly reminding to germ cells, and are organized into wide cell fields, that are cut up by fibrotic hedges with lymphocyte infiltration. B (OCT3/4; 200x): Tumor cell nuclei show positivity to OCT3/4 (transcription factor of embryonal stem cells). (From the archive of the Semmelweis University, 2nd Department of Pathology–collection of Ildikó Szirtes)
|
Clinically, the affected testicule can be only slightly enlarged, or enlarged to ten times, but at the same time it usually keeps its original shape. This suggests that sheaths are rarely affected. This tumor is definitely sensitive to radiation, 5 year mortality is less than 5%. Survival after 5 years practically means recovery, because in case of ineffective treatment, death occurs within 2 years, but almost in all cases, within 5 years from the discovery.
II./2.5.3.1.: Spermatocytic seminoma
|
 |
Usually it occurs in a later age group (over 65 years) even compared with that typical in seminomas. It is a soft tumour, its cut-surface is yellowish, generally with reddish patterns. Histologically, it is usually constituted of three cell types: the majority is the group of cells similar to seminoma, then large, bizarre, and smaller cells can also be observed, reminding to spermatocytes. In the cytoplasm there is practically no glicogen, and thus the PLAP reaction is much weaker, or negative, and there is no lymphocytic infiltration. Especially, the organization of the chromatin is very similar to that in primary spermatocytes.
This type has to be distinguished from seminoma, because its prognosis is very good, practically does not metastatize. Interestingly, spermatocytic seminoma only occurs in testicules (while the other types may occur extragonadally). Spermatocytic seminoma, although rarely, but may co-occur with unmature sarcoma, in which case it does metastatize.
|
|