| |
III./2.3.: Characteristics of GIST’s
III./2.3.1.: Definitions, introduction
|
 |
The gastrointestinal stromal tumor (GIST)
is the most common mesenchymal tumor of the gastrointestinal tract. GIST originates of mesenchymal progenitors which are cells reaching various levels of phenotypical differentiation in the following directions: (1) Cajal c-kit/CD117-positive interstitial cells , the so-called pacemaker cells of the gastrointestinal tract, SMA-positive smooth muscle myocytes, or CD-34 positive perineural fibroblast cells.
III./2.3.2.: Epidemiology
The estimated incidence of GISTs in the general population is 10-20 patients/million, including incidental minimal tumors (see there). Topographically, 60% of these tumors have a gastric origin (Illustration 1.), 4-5% originates from the duodenum and 20-30 % from the jejunum (Macropicture 1.). Other, less common locations of origin are the following: oesophagus (<1%), gall bladder, colon and appendix (1-2%), rectum (4%). Additional, rare locations are the omentum (Macropicture 2.), the mesenterium and the retroperitoneum; GISTs originated in the above mentioned organs are called extragastrointestinal GISTs.
|

Look at the pictures and evaluate them!
|
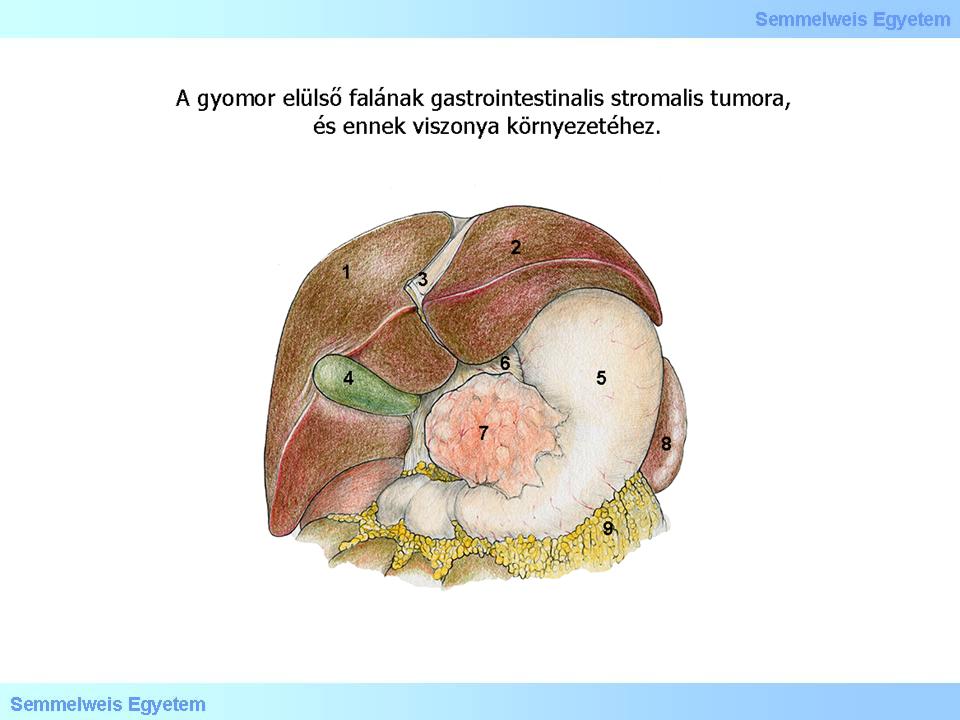
Illustration 1: Gastrointestinal stromal tumor of the anterior gastric wall and it’s relation to its surroundings.
|

Macropicture 1: GIST in the wall of the small intestine. The tumor protrudes from the intestinal wall, grows in outward direction, bulging the outer surface outwards. It is tuberous but has exquisite margins.
|
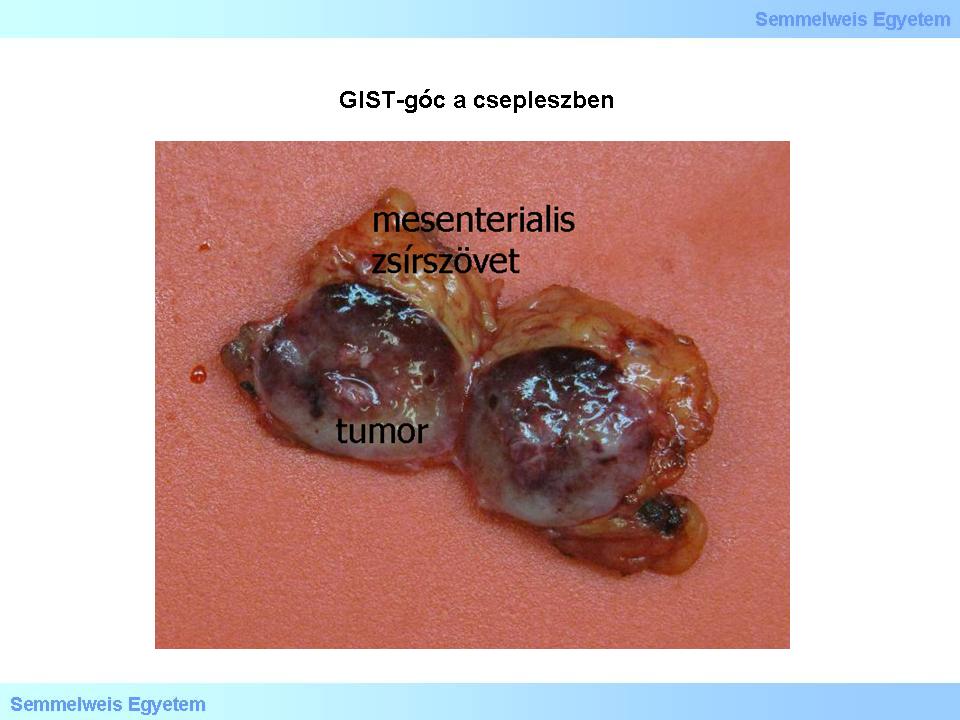
Macropicture 2: GIST-nodule in the mesenterium. Due to the exquisite tumor margins, the GIST-nodule has been cut out with close resection from the mesenterial adipose tissue.
|
GISTs are extremely rare in childhood (<1%). As for age distribution, sporadic GISTs are most common in 60-62 years old patients, however, certain specific syndromes, which are less frequent compared to the sporadic GISTs (e.g. GIST-syndromes, childhood GISTs, see there) has different age incidence peaks. The majority of GISTs are sporadic, only a few families showed germ line mutations of the KIT- (see below), or PDGFRA- (platelet derived growth factor alpha – a tyrosine kinase similar to c-kit/CD117 with the same effect) genes.
III./2.3.3.: Appearance forms, dignity types
|
 |
The GIST spectrum ranges from indolent, incidentally discovered small tumors (tumorlets – see there) to large, in part necrotically disintegrating, metastasizing sarcomas that may reach every possible location. Incidence of the latter type is around 20-30%. According to general observations, even the benign-rated tumors can re-occur, sometimes quite late (after years); late metastases can develop, and the unresected tumor itself shows a slow but gradual growth. Thus nowadays „benign GIST” is an incorrect term: according to common understanding these tumor always has to be considered as tumor with low malignant potential (LMP, borderline dignity).
Other authors are stricter on the issue and consider all GISTs - except the ones under 1 cm size - malignant. As for the location, gastric tumors has a better prognosis than the ones originating from the small intestine. GIST can also be part of a tumor syndrome, e.g. von Recklinghausen’s disease, Carney-syndrome (GIST + paraganglioma + pulmonal chondroma).
III./2.3.4.: Location of metastasis
Most common location of metastases is the liver (around 30%), and the peritoneum. Way of metastasizing is mainly haematogenic. Lymph node metastases are rare, thus lymphadenectomy is usually not done when resecting the primer tumor. Pulmonal, and other metastases outside the abdominal cavity are also uncommon.
III./2.3.5.: Macroscopic characteristics
A mainly circumscribed, roundish tumor node with exquisite margins, fibrous texture, greyish-white colour and dense tact is located intramurally – in submucose, intramuscular or subserose position –
under intact or secondarily abscessed (Illustration 2.) mucosal cover.
The centre of large tumors can necrotize, so that vital tumor tissue is left only in a zone of variable size on the borders. Thus a cave develops with ragged, necrotic inside cut surface, causing the tumor a cystic appearance (Macropicture 3.) Less often the tumor has more than one nodular centre. Mainly gastric GISTs and small intestinal GISTs accompanying neurofibromatosis type 1 (NF-I.) appear in multinodular forms, and so does the typical childhood GIST.
|

Look at the picture and evaluate what you see!
|
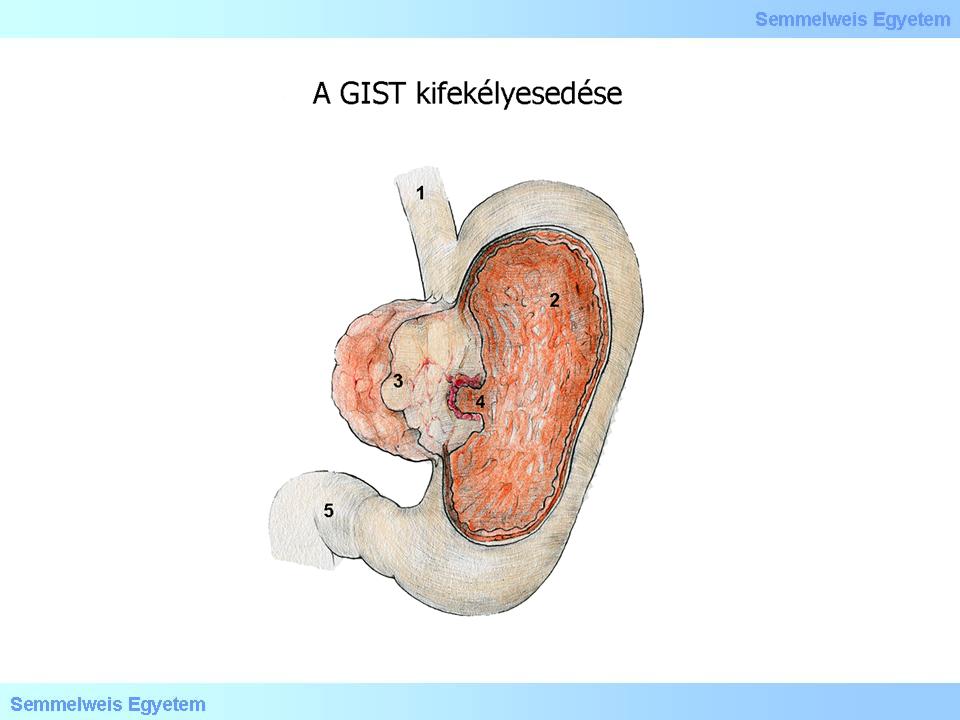
Illustration 2: GIST abscess can be the origin of gastrointestinal bleeding threatening the whole circulation
|
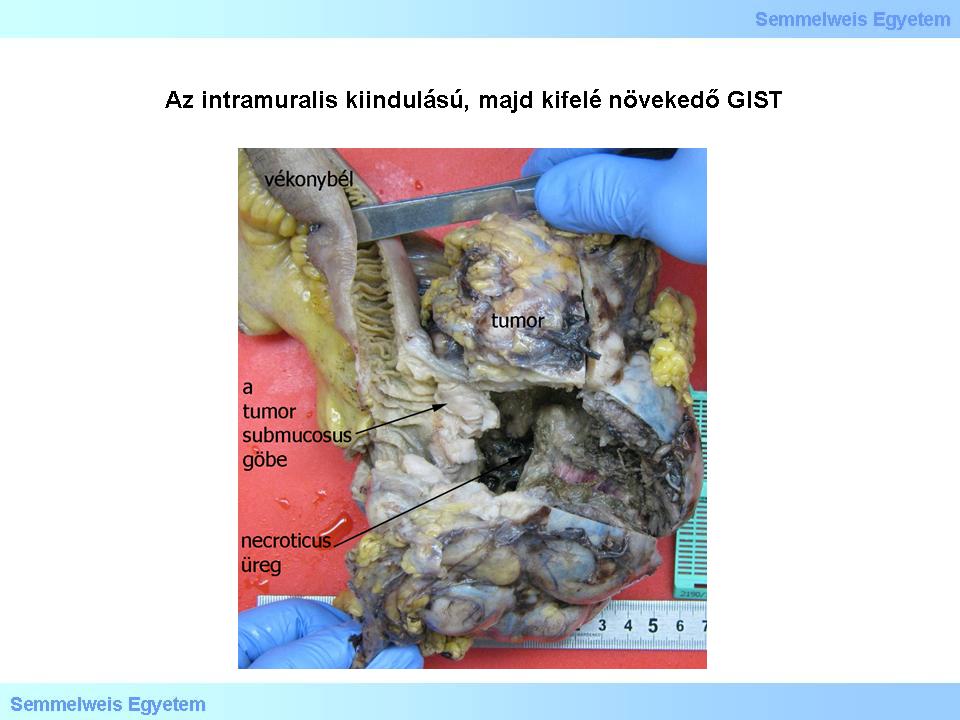
Macropicture 3: The outward-growing GIST with intramural origin contains a large cavern due to central tumor necrosis. The tumor node is circumcised, roundish, borders are exquisite. The outer, vital zones are greyish-white, dense. In the presented case, tumor nodules appear directly under the intact mucosa.
|
III./2.3.6.: Microscopic characteristics
Most GISTs consists of one of the following three tissue types: the most common spindle cell type (Micropicture 1. and 2.), the epitheloid type with broader cell bodies (Micropicture 3.), or the mixed, spindle- and epitheloid cell type.
Other, rarer tissue variants are also described, such as the pleomorph, or giant cell type. The tumor is moderately or extremely rich in cells, which are arranged in bundles. The frequency of proliferating forms is variable, increased proliferating activity can be found in aggressive tumors, so the number of mitoses is the most important factor of risk stratification. Among the rare tissue types, pleiomorph or giant cell GISTs are usually PDGFRA-gene mutants . Lack of either KIT or PDGFRA-mutations are found in the rare cases of anaplastic, so-called dedifferentiated GISTs.
|

Look at the pictures!
|
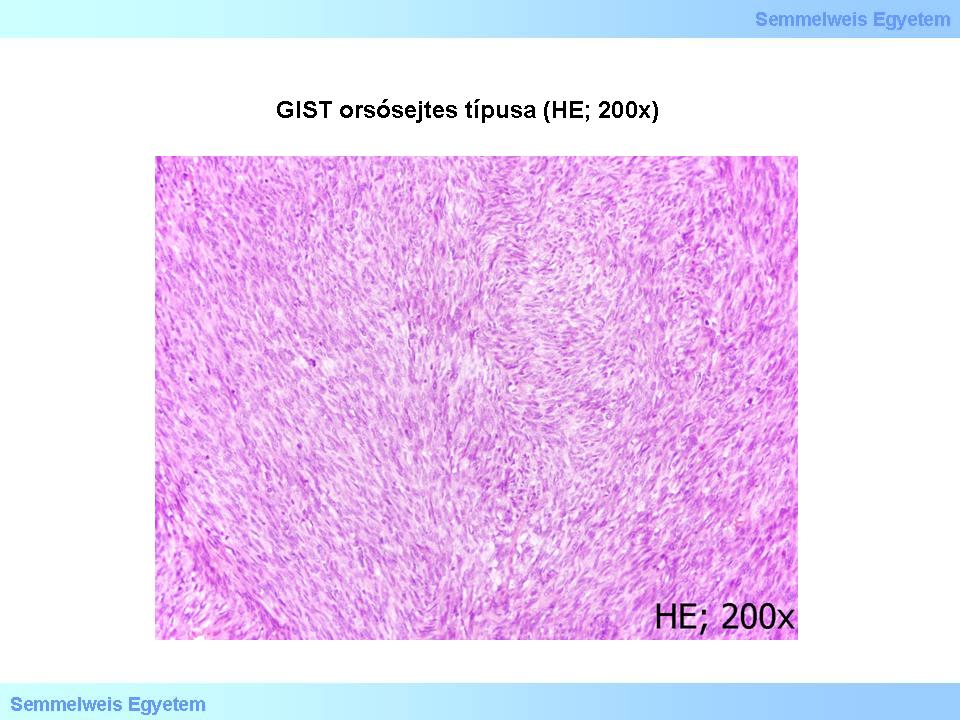
Micropicture 1: Spindle cell GIST (HE; 200x). The tumor is rich in cells, cells are arranged in bundles
|
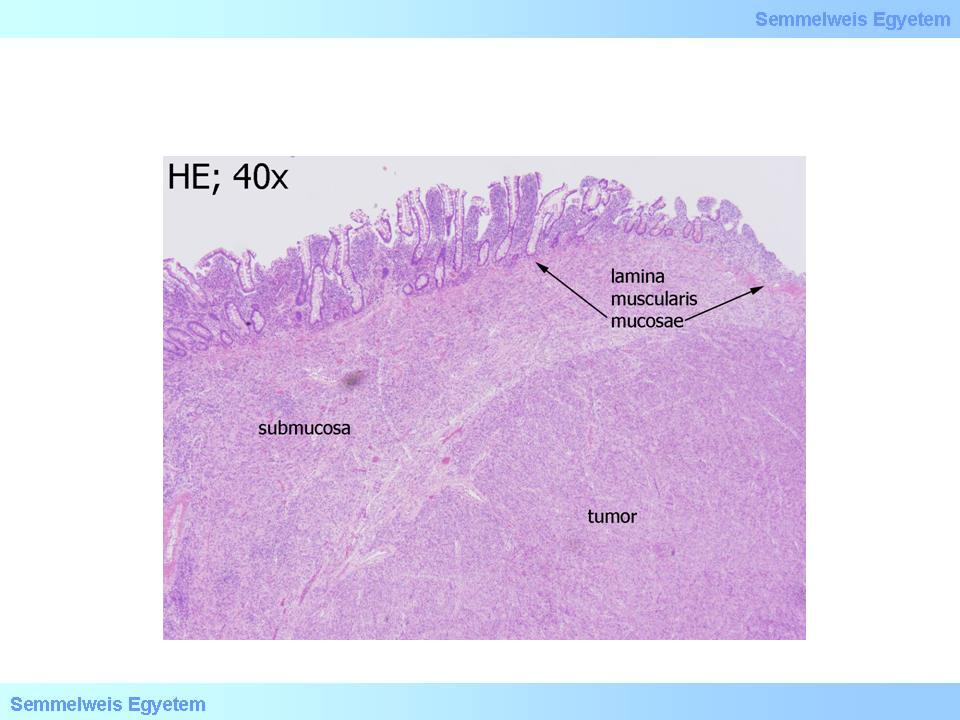
Micropicture 2: Spindle cell GIST (HE; 40x). Intact small intestinal mucosa can be seen above the tumor.
|
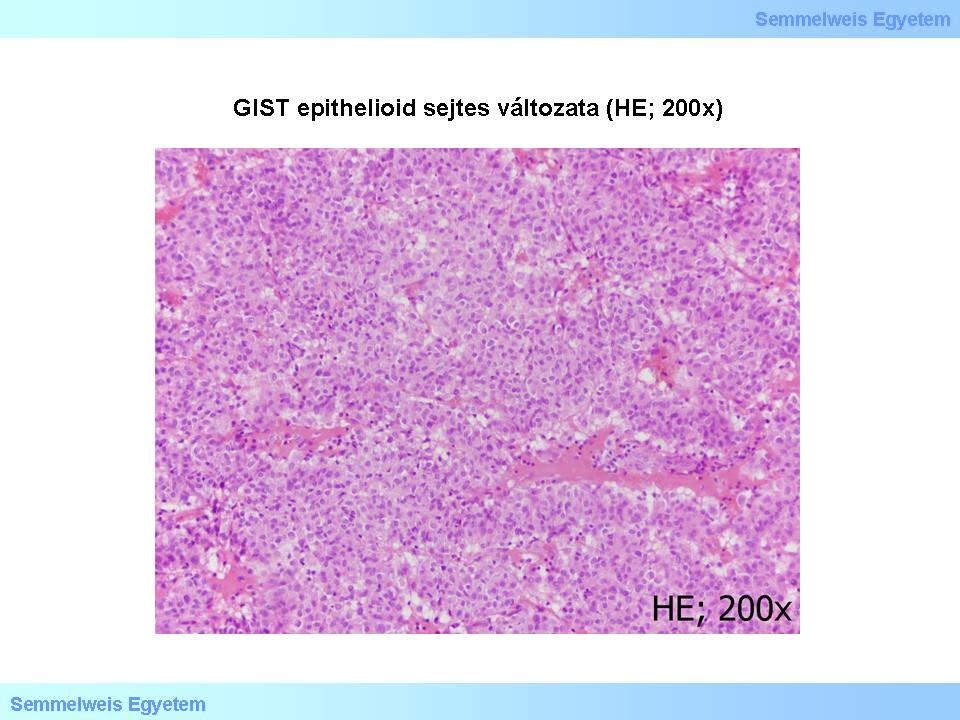
Micropicture 3. Epitheloid cell GIST (HE; 200x). The tumor is rich in cells. Cells are roundish-ovoid, cell bodies are broad, cell margins are exquisite.
|
Seventy percent of the cases are spindle cell GISTs. Cells are arranged in short bundles and vortexes with myxoid-looking stroma in between, sometimes containing bone tissue metaplasia. In some cases the cell arrangements is similar to the Antoni-A type parts of schwannomas (nuclear palisading). A stroma lesion called ’skenoid fibers’is commonly seen in small intestinal GISTs. This consists of elongated, broadly filamentous, actively eosinophilic, hyalonic-fibrillar, high-contrast extracellular collagen globular structures.
About 20% of GISTs are epitheloid and 10 % are mixed types.
III./2.3.7.: Immunreactivity
The c-kit/CD117 is almost always (85%) positivein GISTs , in the spindle cell (Micropicture 4) and epitheloid cell (Micropicture 5) tissue types as well. The CD34 (haemopoetic progenitor stem cell- and endothelial marker among others) is on the average 70% positive: 80% of gastric GISTs, 50% of small intestinal GISTs, and 95% of oesophagus and rectal GISTs. For that matter, c-kit/CD117 can be positive even in a CD34-negative GIST. Smooth muscle markers (SMA, desmin) are positive in 20-40%, but in about one-third of the cases c-kit/CD117 is co-expressed with SMA (smooth muscle actin). Evaluation of myogenic markers requires special attention, as the fragments left from the organ wall’s muscle layers (lamina muscularis mucosae, tunica muscularis propria) cramped by the tumor might be misinterpreted as myogenic tumor parts.
|

Look at the pictures!
|
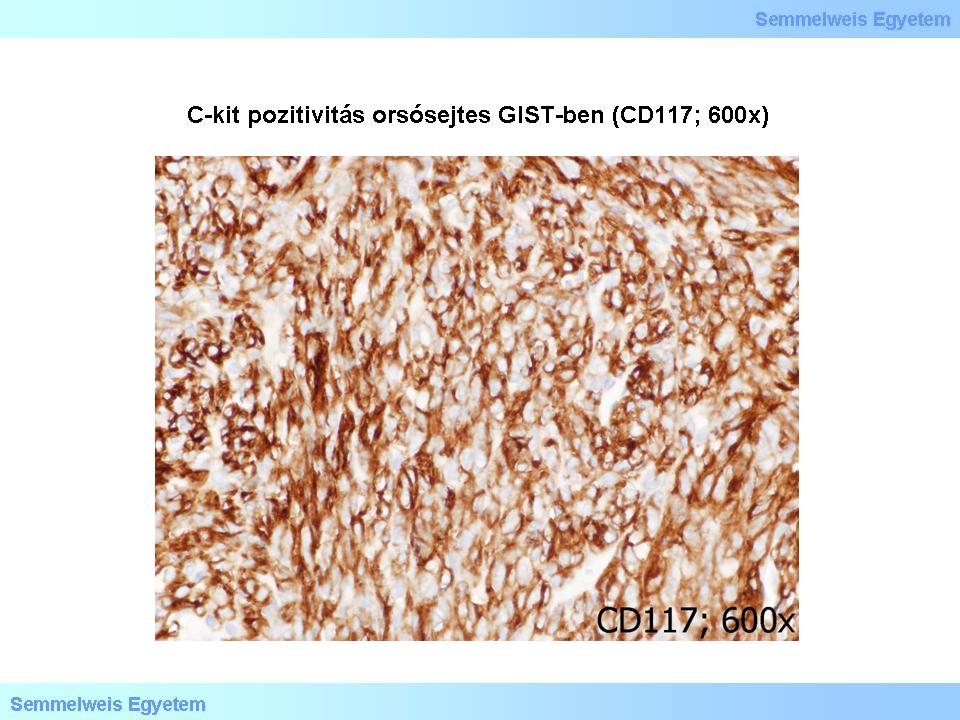
Micropicture 4: C-kit positivity in spindle cell GIST (CD117; 600x).
|
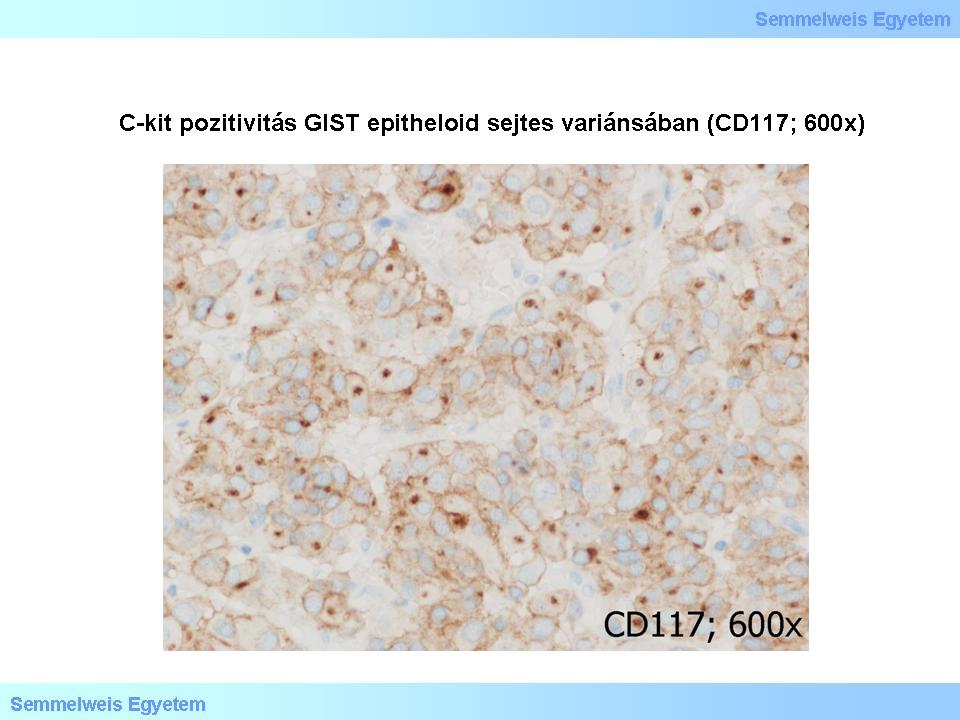
Micropicture 5: C-kit positivity in epitheloid cell type GIST (CD117; 600x).
|
Further, recently discovered GIST-specific markers are protein-kinase C-theta (PKCθ – see below) and DOG1. The latter is coded on FLJ10261 gene, the product of this gene is the chloride- channel protein called DOG1. This reaction is positive in the case of several normal structures, such as Cajal-cells, and surface cells of the gastric mucosa. DOG1 appears to be quite specific to GISTs, irrespectively of the tumor’s KIT- or PDGFRA- (see below) mutational state. The GIST-sensitivity of DOG1 is high, similarly to c-kit. DOG1 positivity is present is extragastrointestinal and metastatic GISTs as well. In summary, the immunhistochemical panel composed by the c-kit/CD117- , CD34-, protein kinase C-theta- and DOG1- markers is a suitable tool in the identification of these tumors (Micropicture 6).
|

Look at the picture!
|
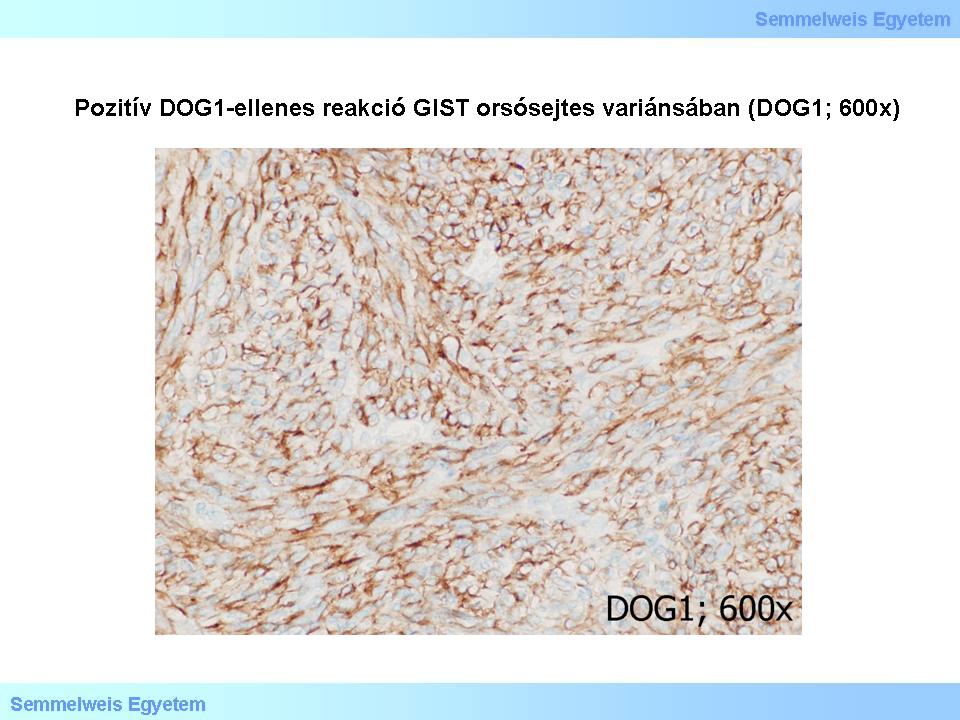
Micropicture 6: Positive DOG1 reaction in spindle cell GIST (DOG1; 600x).
|
III./2.3.9.: Molecular genetic background
|
 |
In the vast majority of cases, KIT gene’s (or the very similar PDGFRA gene’s, see below) activating mutation can be detected in the background of c-kit/CD117 positivity, thus formerly this was believed to be the obligatory explanation of the protein expression phenotype. By now it is clear that the situation is more complicated than that: not only the mutant cases are c-kit/CD117 positive, but the ones without the mutation, the wild type KIT (WT-KIT) can test positive as well. Furthermore, c-kit/CD117-negative tumors can include mutations in both KIT and PDGFRA-genes. Therefore, in cases of c-kit/CD117 tumors, assessment of the mutation state is recommended as a diagnostic help. Syndrome-associated (neurofibromatosis type 1., Carney-syndrome, etc.) and childhood GISTs (see there) can also test positively for c-kit/CD117, even though their KIT and PDGFRA-genes are intact. The cause of oncogenesis is not known in these cases.
The KIT proto-oncogen is located on the long arm of chromosome 4, and it codes the transmembrane tyrosine kinase receptor called c-kit/CD117. In sporadic GIST cases, this gene has mutation lesions mainly in exon 11, less often in exon 9, 13 and 17. The most common (65%) mutation is that of exon 11, which codes the receptor’s juxtamembrane domain. These mutations are mostly in-frame deletions, and some of them (e.g. 557-558 codons) amount to unfavourable prognoses. Other mutation variants: deletion/insertion 557, 559, point mutation: 557, 559, 560, 576 – these amount to better prognoses in gastric GISTs but this correlation is not true in small intestinal GISTs.
The PDGFRA gene has a pathogenetic role in c-kit/CD117-negative GISTs. Similar to the KIT gene, PDGFRA is located on the long arm of chromosome 4 and codes a transmembrane tyrosine kinase receptor type III. Point mutations are present in exon 12, 14 and 18, especially in gastric GISTs. The most common (around 6 %) is the exon 18 deletion, which leads to imatinib resistance. The mutation on exon 12 causes the loss of the ligand binding domain and amounts to imatinib-sensitivity. GISTs with PDGFRA mutations are mainly found in gastric or extra abdominal locations, their tissue often contains epitheloid phenotypes, myxoid stroma and giant cells with multiple nuclei.
Other, additional chromosome deletions can be present and have a correlation with tumor progression; some examples are chromosome 14 monosomy and missing chromosome 22.
|
 |
In summary, activating mutations are present in 90% of GISTs; 85% KIT- , 5% PDGFRA- gene mutations. Currently no mutation can be identified in 10% of the cases. KIT- and PDGFRA-mutations reciprocally rule out each other. Those negative to KIT and PDGFRA as well are called KIT/PDGFRA-wild GISTs or wild type GISTs (WT-GIST). Recently in these cases the BRAF V600E mutation of exon 15 was detected in adults.
III./2.3.10.: Group of c-kit/CD117-negative GIST’s
Even though c-kit/CD117-receptor-tirosin-kinase expression is the most important factor in tumor rating, still there is a group of tumors, satisfying all clinical and morphologic criteria of GISTs, that are c-kit/CD117 negative (10-15% of all the GIST cases). These tumors often originate from the mesenterium, gastric or retroperitoneum, but similarly to the c-kit/CD117 cases, as a matter of fact they can appear in any section of the gastrointestinal tract. Their tissue spectrum is also similar: any tissue type can appear from epitheloid to spindle cell and anaplastic variants, sometimes showing CD34- or SMA-positivity. PDGFRA genealterations are detected in a subset of c-kit/CD117 negative cases (around 35%): exon 18 mutation, the Asp→Val842 missense substitution of the gene product and DIM842–844 amino acid deletion.
III./2.3.11.: Familiar GIST and other GIST-syndromes
|
 |
Family history of GIST cases were described by Nishida et al. in 1998. Mutations found in these GISTs are similar to the ones in sporadic GISTs. Inheritance is autosomal dominant. More than one GIST can develop in the affected members of these families at earlier ages compared to sporadic GISTs, and this can be accompanied by other lesions: perineal hyperpigmentation, great number of pigmented naevi, mastocyte abnormalities (urticaria pigmentosa, systemic mastocytosis).
Even though familiar GISTs cannot be morphologically differentiated from sporadic ones, some authors still presume that – contrary to the sporadic variant –patients having these tumors has an increased risk of metastases regardless of the size and mitotic activity of the primer tumor, and thus they suggest careful and continuous follow-up of the at risk members of familiar GIST families.
Recently the following three syndromes are known to predispose developing more than one GIST:
-
- Carney-triad
-
- Carney-Stratakis syndrome
-
- neurofibromatosis type 1 (NF-1.)
Carney-triad consists of GIST, paraganglioma and pulmonal chondroma, mainly in young woman. These GISTs are pathologically and clinically different from sporadic types: they develop slower, their tissue type is epitheloid, they have a higher risk of metastasizing and give metastases especially into lymph nodes. Neither KIT, nor PDGFRA-mutations were detected in these tumors. Development of Carney-triad-associated GISTs is currently explained by somatic mutation, which means the oncogenesis is regarded sporadic. Nevertheless, these tumors do not, or at least not unambiguously react to imatinib (Glivec) treatment used in sporadic GISTs, and traditional risk assessment schemas are not reliable in prognosting clinical development.
GISTs in Carney-Stratakis syndrome are heritable through the germ line mutation of succinate dehydrogenase (SDH) SDHB, SDHC or SDHD subunit. Clinical features of the syndrome are multifocal GISTs, paragangliomas and pheochromocytoma. Carney-Stratakis-syndrome associated GISTs are mainly gastric, and have epitheloid tissue type.
About 7% of neurofibromatosis type 1 (NF-1) patients has multiple GISTs by means of the germ line mutation of gene NF1. This gene codes the GTP-ase activator neurofibromin protein. NF1-associated GISTs characteristically appear as small, multiple tumors in the small intestinal wall, their tissue variant is spindle cell type loaded with skenoid fibers . The vast majority of these tumors show neither KIT-, nor PDGFRA-mutations, their clinical behaviour is indolent.
III./2.3.12.: Childhood GIST
|
 |
These GISTs are detected in the second life decade, generally at age 13-14,5 years. A pronounced female dominance is present in sex distribution. Tumors appear mainly in gastric location, are often multifocal and characteristically epitheloid tissue types. Although they uniformly produce the c-kit/CD117 protein, very rarely can either KIT- or PDGFRA-mutations be detected.
Tissue and clinical characteristics are surprisingly similar to those of Carney-triad associates GISTs, and some of the patients actually have Carney-triad, thus control examinations for Carney-triad are reasonable in any GIST detected in childhood. Risk stratification schemas used in adult tumors are not suitable for prognosting childhood tumors, as these usually show a favourable prognosis even when metastasizing: tumor-free 5-year survival is around 70%, survival with tumor is around 20%, and tumor-related mortality is around 10%.
III./2.3.14.: Risk stratification of primary GIST’s
According to current guidelines, primary sporadic GISTs risk stratification should be based on the following three factors:
-
1) mitotic index,
-
2) tumor size
-
3) anatomical location (Table 1).
|
 |
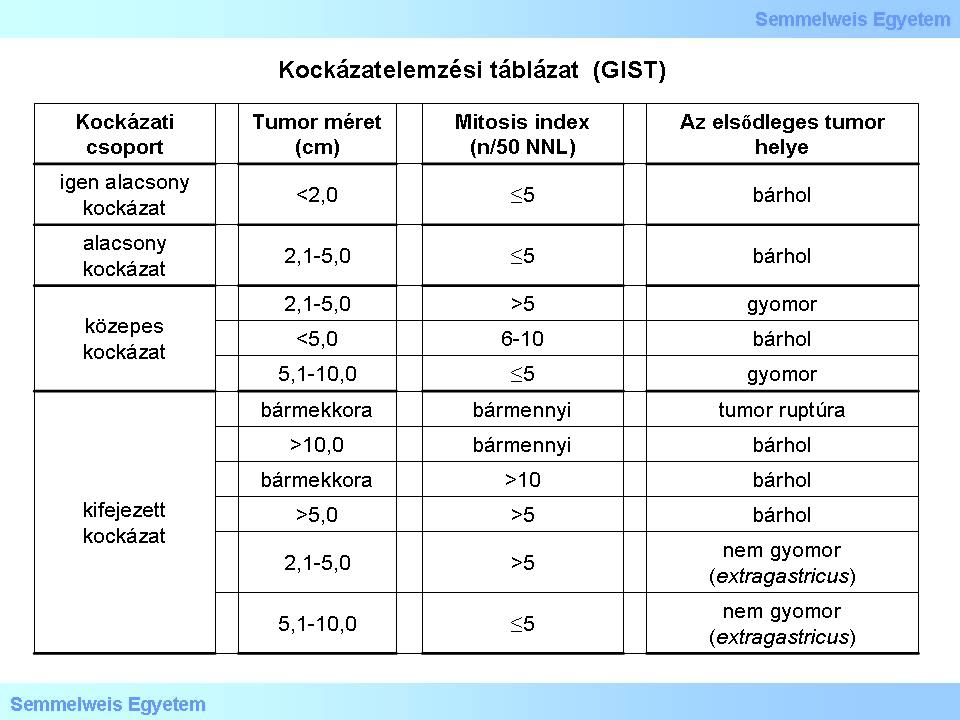
Table 1. Risk stratification table based on the National Institutes of Health (NIH) data. (Joensuu H: Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Human Pathol 2008, 39: 1411-1419.)
|
-
1) Mitotic activity is way the most important prognostic factor. Its value has to be given as the number of mitoses projected to 5 mm of the tumor (this corresponds with 50 HPF’s (high power field) in older microscopes and 20-25 HPF’s in modern, broad view microscopes.
-
2) Tumor size has to be given in centimeters. Its prognostic role is based on the fact that larger GISTs have worse prognoses compared to smaller ones.
-
3) Significance of anatomic location is based on experience: as a rule, gastric GISTs are more indolent than GISTs of any other (extragastric) origin.
The collective effect of the above three factors are shown in the following set of criteria:
|
 |
-
- metastasis risk is low in gastric GISTs not larger than 10 cm and containing not more than 5 mitosis per 5 mm2;
-
- metastasis risk is high in gastric GISTs larger than 5 cm and containing more than 5 mitosis per 5 mm2;
-
- metastasis risk is at least moderate in intestinal GISTs larger than 5 cm, regardless of mitotic activity;
-
- metastasis risk is high in intestinal GISTs larger than 5 cm and containing more than 5 mitosis per 5 mm2;
-
- metastasis risk is low in intestinal GISTs not larger than 5 cm and containing not more than 5 mitosis per 5 mm2;
|
|