| |
I/2.5: Ischemic heart disease syndrome
I/2.5.1: Coronary artery hardening (coronary sclerosis) as a cardiac manifestation of arteriosclerosis
I/2.5.1.1: Introduction
|
 |
Coronary artery system consists of the coronary arteries’ subepicardial main branches, the epicardial subbranches of these, and intramural small vessels originating from the latter which – apart from their minimal subendocardial connections – are all end-arteries. This structure has an important role in the ischemic necrosis of the myocardium (infarction). In terms of blood supply, three large trunks of the coronary artery tree are distinguished: the right coronary artery, the left coronary artery’s anterior interventricular branch, and the left coronary artery’s circumflex branch system.
Variations of coronary artery pathways should also be mentioned, where certain sections of subepicardial coronary artery branches dive into the outer, more superficial layers of the myocardium, and tunnel under a so-called muscular bridge intramurally for a few centimeters, and then turn back to the subepicardial fat tissue. It is interesting, that even when the coronary artery system is very sclerotic subepicardially, these deeply tunneling sections are still relatively intact, which is thought to be due to the supporting role of the elastic muscular bed. This is supposed to protect these vascular sections from intimal microtrauma resulting from the pulsating pressure of the blood flow, thereby decreasing the rate of atherogenic material inflow into the wall.
Unfortunately this favourable protecting effect is lost because at the sites where the vessels go under and leave the muscular bridge, even more severe lesions are seen then elsewhere. Those are the borders between regions with outer support and unsupported regions, and therefore the vascular wall is exposed to hemodynamically greater forces here than usual, and this promotes insudation of atherogenic materials.
I/2.5.1.2: The causes of coronary artery sclerosis and obstruction
|
 |
In 80 to 90 percent of the cases, the causative factor is some form of arteriosclerosis, and the complicated plaques are the most dangerous (hemorrhage, thrombosis). Coronary embolism is rare and congenital developmental disorders e.g. coronary hypoplasia are also infrequent. Coronary artery pathway disorders are also rare, where the coronary artery originates from one side of the aortic root and, passing round the aorta, runs toward the other side of the heart. Other causes include inflammations, autoimmune vascular diseases, arterial wall spasm (Prinzmetal’s ‘variant’ angina), and muscular bridge formation.
I/2.5.1.3: Classification of coronary artery hardening
|
 |
1. As per the extension within the affected vessel, we talk about central, peripheral or diffuse coronary artery disease. In the central form the sclerotic stenoses are located in sections close to the origin of the artery, where the diameter is at least 0.1 cm. So this is not a certain vascular section with a defined length but rather a technical-functional border, for it is known that a vessel is only suitable for bypass above this caliber. Peripheral coronary artery disease, which is also called small vessel disease, affects vessels with a caliber less than 0.1 cm, and they are not suitable for coronary bypass surgery. It is relatively rare. Diffuse coronary artery disease affects both central and peripheral sections of the vessels; this is the most frequent form. Success of coronary artery bypass grafting (CABG) is also questionable here.
2. As per the number of affected coronary arteries, so-called single vessel disease, two vessel disease, and three vessel disease are known. In single vessel disease the angina is localized to the chest, and the symptoms last for less than 3 years, while in three vessel disease angina is usually present for more than 3 years, and the pain irradiates into the arm, nape and back.
3. As per severity of the affected vessels’ disease, there is mild, moderate and severe coronary artery sclerosis. In mild coronary artery hardening the stenosis is less then 50 percent of the original cross-section, in moderate sclerosis it is 50 to 75 percent of the original cross-section, while in severe coronary artery disease it is over 75 percent. In moderate vascular disease, ECG taken in rest doesn’t show any alterations yet, while in case of severe stenoses, effort ECG is pathological indicating the failure of coronary arteries.
I/2.5.1.4: Therapeutic reconstruction of coronary artery blood flow
Reconstruction of coronary circulation can be attempted medically (antithrombotic or vasodilating agents, etc.) or surgically. Open heart surgery is considered a classic intervention today to attempt to go round the stenosis using a vascular graft (CABG – coronary artery bypass grafting) and thus supply the myocardium with blood (macroscopic image No.3.). Most usually the great saphenous vein is used for this purpose which has an easily approachable long section on the shin and can be removed without any significant consequences (microscopic images No.1-3.), but the internal mammary artery can also be used which runs from the subclavian artery on the internal surface of the thoracic wall toward the diaphragm, and sometimes a properly chosen section of the radial artery is transplanted.
|

Study the images carefully!
|
macroscopic image No. 3: Double vascular bypass on the first interventicular and on the circumflex branch. A small brown spot indicating total occlusion (anastomosis thrombosis) is seen in the distal anastomosis of the bypass graft running to the interventricular branch. (From the museum of the 2nd Institute for Pathology, Semmelweis University – collected by Tibor Glasz)
|
microscopic image No.1: (HE; 40x) Bypass graft anastomosis stenosis cases (I.) – anastomosis-thrombosis. During anastomosis formation a part of the myocardium, which is located only a few tenths of a millimeter from the anastomosis site, was drawn into the lumen of the anastomosis by the suture, and it led to anastomosis thrombosis (From the collection of the 2nd Institute for Pathology, Semmelweis University – collected by Tibor Glasz).
|
microscopic image No.2: (Hart van Gieson; 40x) Bypass graft anastomosis stenosis cases (II.) – plaque rupture. The coronary plaque ruptured due to the imposition caused by the intervention, and the rupture was complicated by thrombosis (From the collection of the 2nd Institute for Pathology, Semmelweis University – collected by Tibor Glasz).
|
microscopic image No.3: (Hart van Gieson; 40x) Bypass graft anastomosis stenosis cases (III.) – anastomosis rupture. The bypass graft is partially torn off from the recipient coronary artery, that is suture insufficiency formed with surgical anastomosis stenosis (From the collection of the 2nd Institute for Pathology, Semmelweis University – collected by Tibor Glasz).
|
|
 |
The minimal invasive method called interventive cardiology gains more and more ground recently. During the intervention a a catheter is introduced into a larger peripheral vessel (e.g. femoral artery) which is used as the entry gate, and this catheter carries a head which is selected for the actual purpose: an inflatable balloon which serves to flatten the plaque causing stenosis in case of angioplasty; a metal device appropriate for plaque removal in endarterectomy, the stent itself (a tubular metal net that stretches upon release – maccroscopic image No.4) in stent implantation. An umbrella term for all these minimal invasive interventions is percutaneous coronary intervention (PCI).
|

Study the image!
|
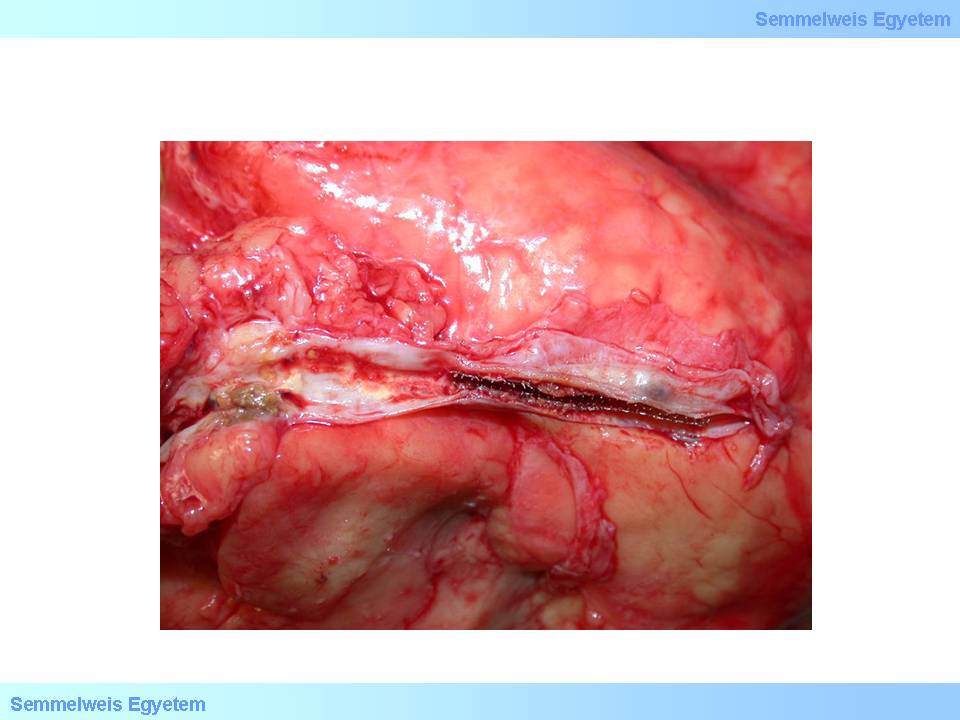
macroscopic image No.4: The left circumflex coronary branch after stent implantation. (From the collection of the 2nd Institute for Pathology, Semmelweis University – collected by Tibor Glasz).
|
I/2.5.2.:Ischemic heart disease
I/2.5.2.1: Concept definition and initiative notes
|
 |
Ischemic heart disease is the name of all clinicopathological phenomenons caused by the myocardium’s insufficient blood supply and the consequent myocardial damage.
Its former name is coronary heart disease.
I/2.5.2.2: Causes of ischemic heart disease
The causes of ischemic heart disease as insufficient myocardial blood supply may be:
-
1. coronary
-
2. and not coronary (extracoronary)
|
 |
1. Coronary causes may be in the level of subepicardial main branches and/or intramyocardial small vessels. Of the diseases of the subepicardial main branches the most common is stenosing coronary sclerosis (>90%!) and its complications (so-called plaque complication: usuration with thrombosis or rupture with hemorrhage); dissection is less frequent (microscopic image No.4); embolism is very rare; and so is the so-called ‘steal’ syndrome (e.g. myocardial arteriovenous malformation, coronary-subclavian-steal syndrome in case of internal mammary artery bypass). The intramyocardial small vessels’ disorders include small vascular spasm, perivascular fibrosis, amyloid protein deposit (so-called obstructive intramural coronary-amyloidosis), and platelet aggregation disorders. Relative myocardial oxygen deficit…[fragment]
|

Analyze what you see on the image!
|
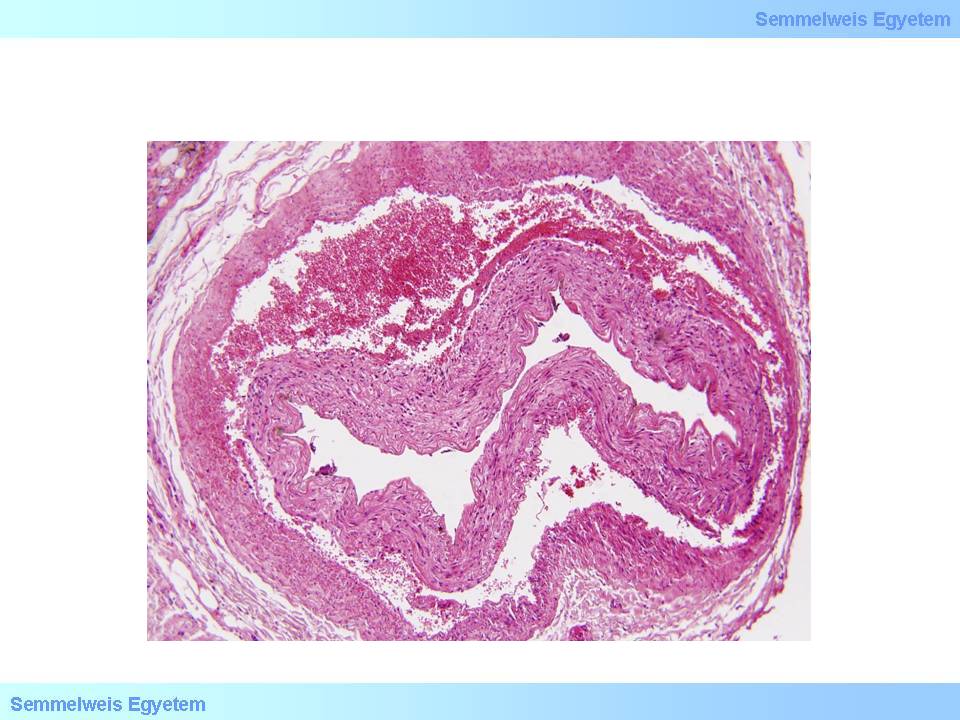
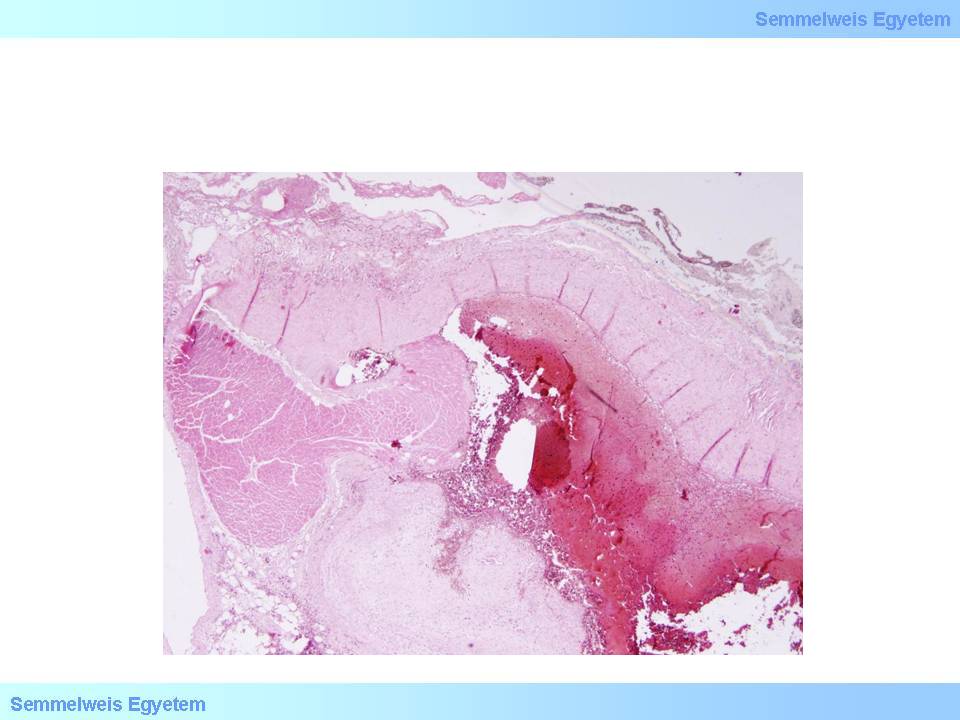
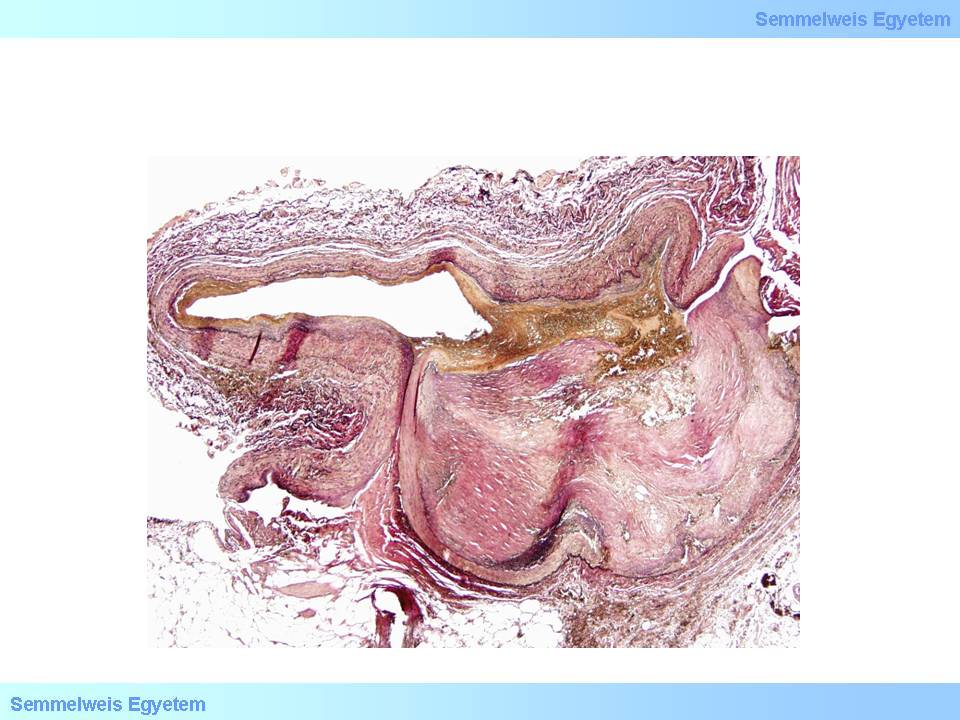
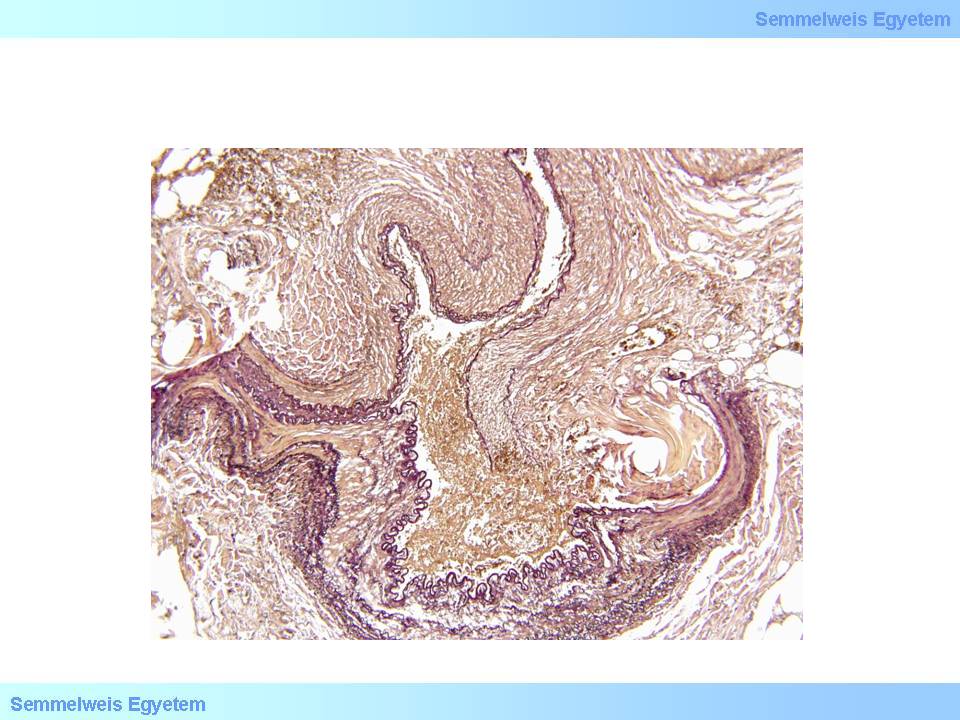
microscopic image No.4: (HE; 40x) Coronary dissection.
|
In case of vascular wall dissection, the mechanism of occlusion is that inner wall layers that separate from the outer layers, jam like a rag into the vascular section distally from the dissection, while blood accumulates and stops in the pseudolumen which was formed between the two layers. It is characteristic of dissection that it can extend from the site where it was formed to even plaque-free distal sections, because there is not much resistance for the blood flow after it has ‘found its layer’ in the vascular wall until inner layers jammed into the lumen stuff the vessel so much that all flow gets tamponaded. That’s how for instance aortic root dissection extends to the coronary arteries. If no such tamponade is formed, the dissection might be stopped by some more resistant vascular wall area (e.g. scarred plaque), or the blood might find a way through another rupture towards the real lumen or out to the perivascular soft tissue (From the collection of the 2nd Institute for Pathology, Semmelweis University – collected by Tibor Glasz).
2. Extracoronary causes include myocardial overgrowth (hypertrophy, the heart weight is over 500g: the myocardial mass becomes unproportionally great for the cooronary artery system to be supplied); stenosis of the aorta or aortic valve (blood gets to the coronary arteries only in decreased volume and with extra muscular work); anemia (the number of oxygen-carrying cells decrease); shock (acute decrease in the amount of the medium transporting oxygen); low pO2 in the air (the amount of available outer oxygen decreases: e.g. in high mountains); pneumonia (decrease in the respirating surface capable of oxygen uptake); increased oxygen need of any origin (e.g. increased physical effort when coronary arteries which are narrowed in various extent suddenly become relatively insufficient).
I/2.5.2.3: Clinicopathological syndromes of ischemic heart disease
Acute manifestations include
|
 |
-
a) angina pectoris,
-
b) sudden cardiac death, and
-
c) heart attack (myocardial infarction).
The chronic clinicopathological form is (iv) chronic ischemic heart disease (chronic ischemic cardiopathy).
|

Study the content of the reference!
|
First it was described by Heberden in 1768 (see the text of his lecture below). In its stable form pain emerges relatively predictably, upon physical effort and in proportion with the effort. It usually accompanies severe coronary sclerosis where well-developed intercoronary anastomoses and collateral circulation through them prevent infarction necrosis of the area but ischemic pain emerges in the myocardium due to slowly developing stenosis of the main supplying artery. In unstable angina the pain attacks occur unexpectedly, even in rest; and this form is considered a preinfarction state. In Prinzmetal’s angina (Prinzmetal, 1959) pain is provoked by transient muscular spasm of the vascular wall, even with healthy coronaries; the attacks start in rest and cease spontaneously.
|
 |
Its incidence in the western world is 30 death/week/1 million people. The heart disease in its background is very often (75%) severe coronary artery hardening. Its further causes include: extracoronary heart disease (cca. 20%) like electromechanical instabilities (sick-sinus-disease, AV-node disease, WPE syndrome, ventricular fibrillation, bradycardia) or structural disorders (cardiac defects, cardiomyopathies, infective endocarditis, myocarditis, artificial valve functional disorder, myocardial rupture). In 5% of the cases there is no detectable cause of death. Idiopathic ventricular fibrillation belongs here which is also called ‘mors sine materia’; it is caused by a heredofamilial molecular genetic flaw and is characterized by damage of the heart’s electric stability. Coagulation myocytolyses are histologically detectable in 67 percent of the cases; sympato-adrenal hyperactivity, stress situations, and physical efforts play a role in their development.
|
 |
One condition that can lead to sudden cardiac death is Adams-Stokes syndrome, which consists of sudden cardiac arrest of variable length (lasting from a few seconds to a few minutes) and desorientation which is proportional with the severity of the consequent cerebral circulatory disorder. Its symptoms are rapidly developing weakness, dizziness, and loss of consciousness. Physically, pulse is not palpable, there is no systole, no heart sounds, and because there is no circulation, the blood pressure decreases rapidly, and tonic-clonic seizures occur. Life expectancies depend on the duration of the circulatory crisis, and in severe cases, it may lead to death. The attack’s duration is variable: ‘forme fruste’ lasting for a few seconds causes moderate dizziness; 10 seconds of duration causes facial pallor, loss of consciousness, muscular clonus, and pupil dilation; 20-40 seconds of circularory arrest causes tonic seizures, cyanosis, and sphincter dysfunction (miction, defecation); and in case of an attack lasting for over 3 minutes the odds of survival are really low.
In most of the casesthere is ischemic heart disease in the background, especially acute myocardial infarction and ventricular fibrillation. The other causes may have cardiac origin (rheumatic myocarditis, cardiomyopathy, heart tumors, arrhythmias, etc.) or extracardial origin (metabolic alterations, electrolite disorders, drug effect, thyreotoxicosis, carotic sinus hyperesthesia, etc.). Arrhythmias causing Adams-Stokes syndrome (so-called electric catastrophes) can formally be hypodynamic disorders (complete AV-block, sinus bradycardia accompanying partial AV-block) or hyperdynamic disorders (ventricular fibrillation as a feared complication of myocardial infarction, paroxysmal ventricular tachycardia).
I/2.5.2.3.3: Heart attack (acute myocardial infarction)
|
 |
Heart attack belongs here didactically, but due to its importance, it deserves a separate chapter as such an ischemic insult of the myocardium that leads to definitive and irreversible morpholological alterations, namely acute necrosis of the working heart muscles.
I./2.5.2.3.4: Chronic ischemic heart disease (so-called chronic ischemic cardiopathy)
|
 |
In 40 percent of all ischemic heart diseases the cause of death is chronic ischemic heart disease. This is the umbrella term of all characteristically and primarily chronic ischemic myocardial damages, including the so-called chronic heart attack. The latter is actually always a transformation of an acute infarction site into scar tissue, thus in this case the nature of ischemic infarction is acute and not chronic, and it gets to its chronic form not by ischemia but organisation. The leading cause of chronic ischemic heart disease is severe coronary artery hardening.
Its other causes might be: left ventricular hypertrophy (heart weight >500g), aortic hypoplasia, and coronary hypoplasia; all of these cause permanent myocardial hypoxia. Macrooscopically it can be characterized by atrophy, normotrophy or hypertrophy (macroscopic image No.5). The histological image is kaleidoscopic: atrophic and compensatorically hypertrophic muscle fibers, microinfarctions (<10mm), focal fibroses and – mainly subendocardially – myocytolytic foci can be seen. Forms of myocardial damage are coagulation necrosis and one-cell necrosis (so-called myocytolysis). Coagulation necrosis is like what we see in infarction; atonic muscle fiber necrosis (irreversible relaxation), fiber expandation and waving occurs due to inner ventricular pressure (so-called wavy fibers).
|

Study the image!
|
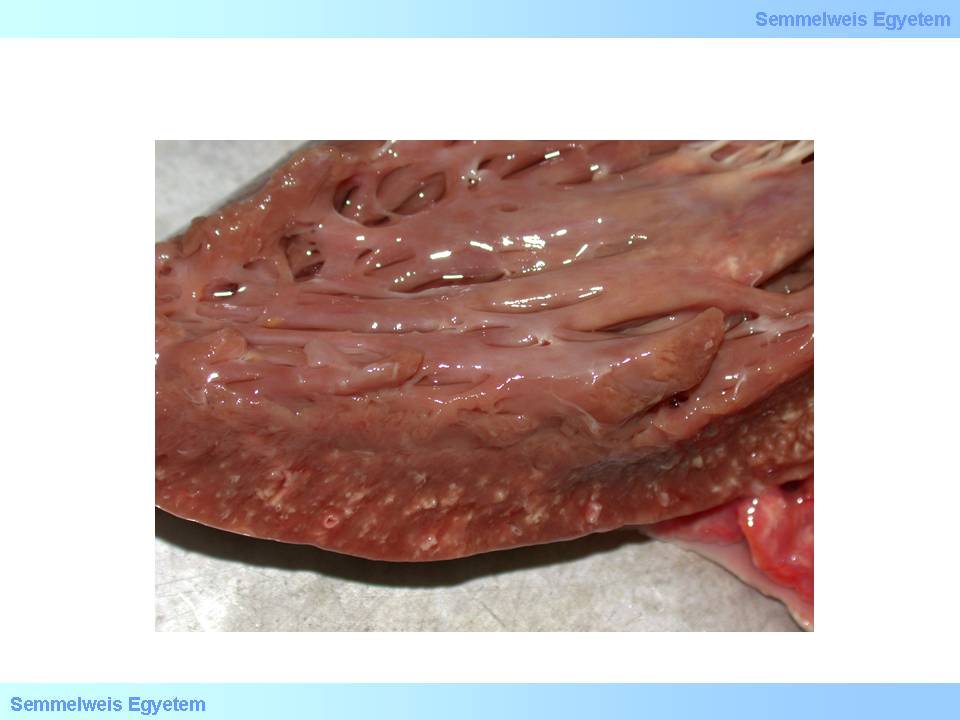
macroscopic image No.5: Myocardial fibrosis, on the ground of ischemic heart disease: there are numerous pointlike whitish scar-foci in the myocardium (From the archives of the 2nd Institute for Pathology, Semmelweis University – collected by Attila Kovács and István Kenessey).
|
In colliquation myocytolysis sarcoplasm dissolvation is the main feature while the reticular myocardial scaffold remains intact and the tissue looks like a fiber net. There is typically no imflammatory reaction, the scarring-like final state is rather the result of sarcolemmal and fibery scaffold collapse and condensation than active fiber formation. Coagulation myocytolysis is less frequent, and the main feature here is tetanic cell necrosis in irreversible contraction because of metabolic reasons. This image is dominant for instance after hormonal effects of pheochromocytoma, heart transplant, or electrocution (microscopic images No.5A-B).
|

Study the image!
|
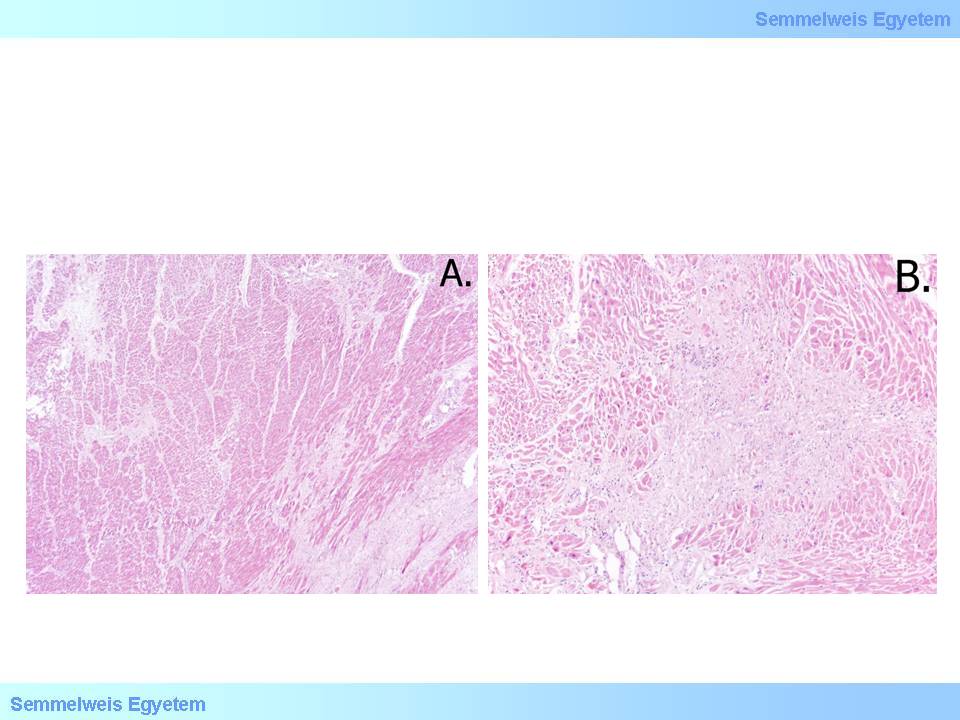
microscopic images No.5A-B: (HE; A: 40x, B: 100x) In chronic ischemic heart disease interstitial fibrosis (A) and small scarred foci of old microinfarctions (B) developed among the intact myocardial fibers. The sizes of the latter don’t reach 1 cm. (From the collection of the 2nd Institute for Pathology, Semmelweis University – collected by István Kenessey).
|
I/2.5.3: Myocardial infarction (heart attack, myocardial necrosis)
I/2.5.3.1: Introduction
|
 |
Acute myocardial infarction is total tissue necrosis of a demarcated myocardial (blood supply) area which is caused by an acute oxygen supply disorder which is beyond the heart’s ischemic tolerance, and develops in a short time
Chronic myocardial infarction is a scar tissue over 1 cm in the myocardium that develops by the organisation of an acute infarction site.
Epidemiologically, i infarction is frequent in patients between 70-80 years of age n the more developed European countries. However, in Hungary infarction is typical one and a half decades earlier, below the age of 65. Acute myocardial infarction incidence and mortality is 2.5 times bigger in our country then the average image in the EU. Besides, it is a well known data that women suffer infarction about 8 years later then men, because atherogenesis accelerates in them only after menopause. In the male population of the same age, atherogenesis starts many years earlier, and by this age it often results in significant arteriosclerosis.
I/2.5.3.2: Causes of myocardial infarction
|
 |
Causes of myocardial infarction are in the same time the causes of oxygen supply disorder too. The most frequent cause is severe coronary artery hardening, especially so-called complicated – ruptured, thrombotized – unstable plaques . Coronary artery dissection (microscopic image No.1P-4) is less frequent, and might also be a consequence of coronary sclerosis. In the background of the latter might be Erdheim’s cystic medial degeneration as a part of Marfan’s syndrome. It is interesting that myocardial infarction can occur even in case of moderate coronary sclerosis if it is accompanied by some ischemic risk factors. The two conditions complicate each other, and such a hypoxic condition develops in the supply area of the not yet critically narrowed artery which leads to ischemic necrosis. Such complicating ischemic risk factors include pneumonia, physical effort, low pO2 in the air, anemia, or serious stress situation.
I/2.5.3.3: Extension of myocardial infarction
I/2.5.3.3.1: Vertical extension of myocardial infarction
Based on the depth (vertical extension), myocardial infarction can be
|
 |
-
- subendocardial, or
-
- transmural.
Subendocardial myocardial infarction confines to the inner (subendocardial) layers of the ventricular wall.
Subendocardium is the myocardium’s strategy zone. This is the site of the whole myocardium with the highest interstitial pressure, because as a layer directly neighbouring the ventricle it has to function under pressure conditions similar to those of the ventricle. It means the highest hemodynamic pressure load of the whole body during systole and leads to total compression of the small vessels during heart contractions, and consequently the actual cessation of blood flow.
This area gets blood supply only during diastole when pressure increases in the coronary system due to the aortic root’s and coronary arteries’ wind box function, and this overcomes the subendocardium’s interstital pressure which transiently decreases to the diastolic value, and therefore the innermost myocardial small vessels’ lumens open for the blood flow. In the same time, this layer is the farthest away from the large epicardial coronary branches whish are the sources of blood supply. We also know that the small vascular bracnches that come here from the epicardium are practically end-arteries, therefore – except for some vascular connections right below the endocardium – there is no way of substitution from the neighbouring areas in case of these vessels’ circulatory disorder.
So, the subendocardium is physiologically in a positively vulnerable situation, since it is the myocardium’s most loaded zone, it is the furthest away from the blood flow source, its blood supply is intermittent, and its collateral connections which could be used for replacement in case of any circulatory disorder, are scarce. It is obvious therefore that in coronary diseases the subendocardium is the first to get damaged. Macroscopically, subendocardial infarctions show a map-like, irregular ischemia in the inner layers of the myocardium with partly blurred outline spreading in a sheet-like pattern. Reparation starts early, on the first postinfarction week – since the necrotic layer is relatively thin – and subendocardial scar tissue forms from the richly vascularized granulation tissue soon. By means of the so-called wavefront mechanism, subendocardial infarctions may develop into transmural infarctions.
|
 |
In transmural infarction tissue necrosis affects the whole thickness of the muscular wall, from endocardium to epicardium. In this case, ischemic insult is so severe that beyond the subendocardium – which has strategic significance – the whole depth of the heart’s muscle wall gets necrotic. The more extended damage is accompanied with more severe and varying complications.
It follows that there is no such thing as an isolated subepicardial infarction, that is, an infarct confining to the outer myocardial layers, since its formation is nonsence both anatomically and pathologically.
I/2.5.3.3.2: Horizontal extension of myocardial infarction
As for the sheet-like (horizontal) extension of necrosis, both subendocardial and transmural infarction might affect one, two, or parts of the three main myocardial support areas (areas of the right coronary artery, the circumflex branch, and the anterior interventricular branch). Topographically the lesions are called:
|
 |
-
- anterior,
-
- lateral
-
- or posterior wall
-
- septal, or
-
- apical infarctions.
In case of larger extension we use some combination of the above names (e.g. antero-septal infarction.
|
 |
Circular ischemic necrosis affecting all three main supply areas is very rare, and – although its pathomechanism is the same as that of infarctions – in a strict point of view – by definition – it isn’t an infarction, but rather global ischemic necrosis of the myocardium, because the necrosis doesn’t confine to a supply area but extends to the whole heart. In these cases the cause is usually not coronary but some more general oxygen supply disorder: e.g. pneumonia, low ambient oxygen concentration, anemia, etc.
In case of anterior wall myocardial infarction the cause is some stenotising/occlusive event in the anterior interventricular branch (left anterior descending – LAD). It usually affects the apical part of the anterior wall and the anterior two-thirds of the ventricular septum. 40 to 50 percent of all infarctions develop here.
In case of posterior wall myocardial infarction the cause is some stenotising/occlusive event in the right coronary artery (RCA) or the circumflex branch (RCX). These infarctions are especially extended if the right coronary is the dominant artery in the heart’s blood supply. Topographically, it affects the left ventricle’s posterior wall, the posterior third of the ventricular septum and sometimes the preseptal part of the right ventricle’ posterior wall. 20 to 30 percent of all infarctions develop here.
In case of lateral wall myocardial infarction the cause is some stenotising/occlusive event in the circumflex branch (RCX) or the marginal branch. 15 to 20 percent of all infarctions develop here.
In case of circular myocardial infarction the cause is some stenotising/occlusive event in at least 2 or 3 main coronary branches within a few hours; it is very rare.
In case of ventricular septal infarction the cause is some stenotising/occlusive event in some of the branches supplying the ventricle; it is also very rare. It is important that even a very small infarction focus can be lethal especially in the upper third of the septum if it affects an important part of the electrical conduction system (bundle of His); the consequent total electromechanical dissociation leads to so-called malignant arrhythmias. Along with electrical conduction disorder, transmural septal infarction also threathens the patient’s life expectancies with pump function disorder, and in case of myocardial rupture, also with left-to-right shunt formation between the ventricles (post-infarction ventricular septal defect).
I/2.5.3.4: Developmental stages of myocardial infarction (post-infarction periods)
|
 |
-
- 30 minutes: Submicroscopic alterations that primarily occur as a result of ion and water flow between the compartments bordered by membranes within the cell, and manifest in e.g. mitochondrial swelling or rough granulation in the cytoplasm. Serologically these alterations lead to elevation of serum Troponin T and Troponin I levels which jump into the pathological range 6 hours after the onset of symptoms and are specific markers of myocardial damage.
-
- 5-6 hours: Microscopically, fibre swelling is seen with intact striae. The nuclei are pale, puffed-up, and sometimes fragmented or lobular. The interstitium is still unchanged. There is no macroscopic alteration yet.
-
- 15 hours: Microscopically, necrotic muscle fibers are elongated, thin, and undulated. The first macroscopic alterations occur: pallor and mild swelling in the affected myocardial area (macroscopic image No.6A). If coronary blood flow starts again (reperfusion) the already necrotic area might get hemorrhaged (macroscopic image No.6B).
-
- 36 hours: Macroscopically the medial infarct areas are mildly yellowish. Hemorrhagic demarcation zone forms at the edges (macroscopic image No.7A-B, microscopic image No.6).
|

Study the image and analyze what you see!
|
macroscopic images No.6A-B: A: Pallor of the necrotic area is the earliest post-infarction alteration. B: Hemorrhagic infarction in the heart is always a result of secondary bleeding; it develops when the circulation restarts (as a result of endogenic thrombolysis or antithrombotic medical therapy) too late and the blood flow arriving to the area finds afunctional, necrotic tissues.
|
macroscopic images No.7A-B: Later the necrotic area – as it becomes alien to the body – demarcates from its living environments. Hyperemic border is the earliest macroscopic form of demarcation.
|
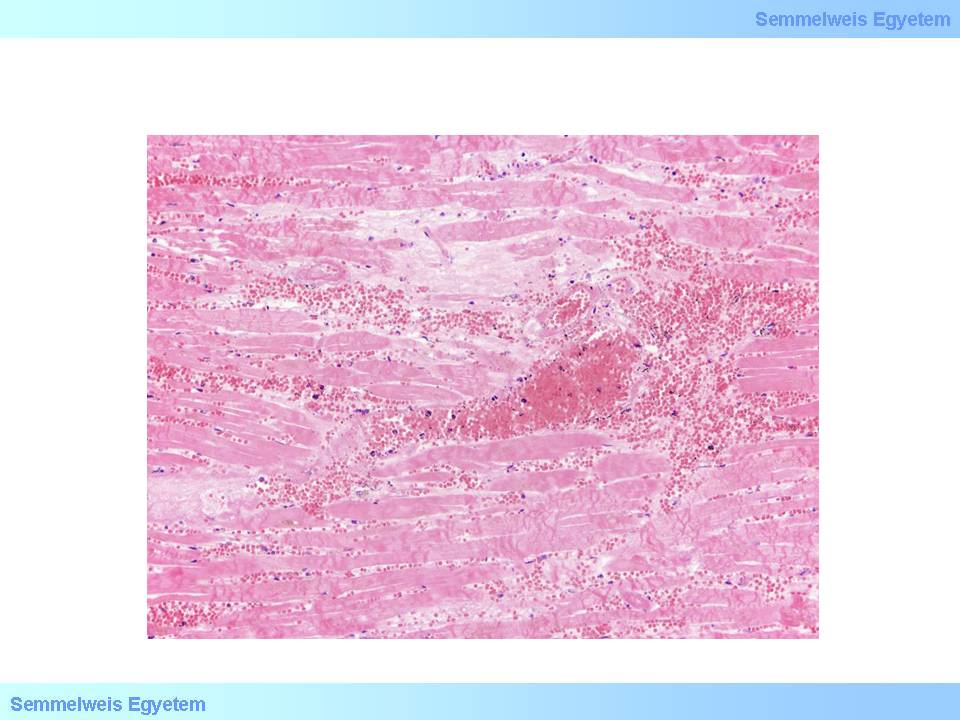
microscopic image No.6: (HE; 100x) Microscopic image of secondary hemorrhage in acute myocardial infarction. Several acute ischemic necrotic signs are seen in the muscle fibers: disappearance of striae, thick contraction bundle formation, lack of nuclear staining, and sarcoplasm homogenization. The interstitium is filled with extravasated red blood cells.
|
-
3-4 days: Macroscopically the infarction site is clay-yellow, and the demarcation zone with map-like irregular pattern at the outline (macroscopic image No.8) is getting sharper. Microscopically in the same time (on post-infarction day 1-5), muscle fibers lose their striae, the sarcoplasm becomes homogeneous and hypereosinophilic and nuclear staining decreases. Interstitial connective tissue fibers become fragmented. Increasing granulocytic inflammatory infiltration appears among the necrotic fibers.
|

Study the image!
|
Macroscopic image No.8: A few days after infarction the demarcation’s outline becomes very sharp and the necrotic area is clay-coloured. The pattern of the outlines are similar to contour lines in relief maps or the beach line of some seashore, that is why we say that these outlines have map-like pattern.
|
-
1 week: Microscopically, macrophage and fibroblast cells start to break down the necrotic tissue area by phagocytosis (macroscopic image No.9). The risk of myocardial rupture and pericardial tamponade is greatest in this phase!
macroscopic image No.9: Due to tissue breaking down by macrophages the necrotic area becomes yellowish-grey after 7-10 days.
|
-
- 2 weeks: Microscopically, reparation signs with granulation starting at the edges. Granulocytes disappear, and their place is taken by lymphocytes, plasma cells and eosinophils. In the same time (post-infarction day 10) the infarction site becomes macroscopically greyish with slowly fading demarcation and shrinkage signs.
-
- 3 weeks: Microscopically, due to fibroblast activity the place of granulation tissue is taken by loose connective tissue, and its fiber content increases in time.
-
- 6 weeks: Micro- and macroscopic end-stage, scar tissue formation (macroscopic images No.10A-C, microscopic image No.7)
|

Study the image and analyze what you see!
|
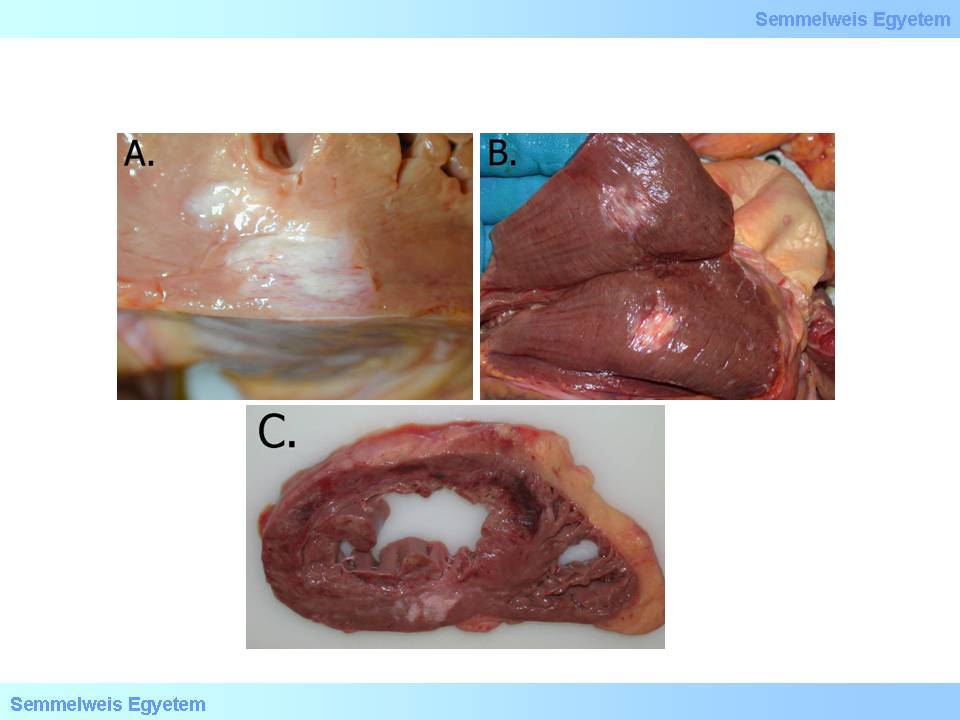
macroscopic image No.10A-C: A-B: Scarring is the end-stage of the organized infarction site. C: Acute hemorrhagic area in the upper part of the image corresponds with acute myocardial infarction, while in the lower part of the image old infarction scar tissue is seen
|
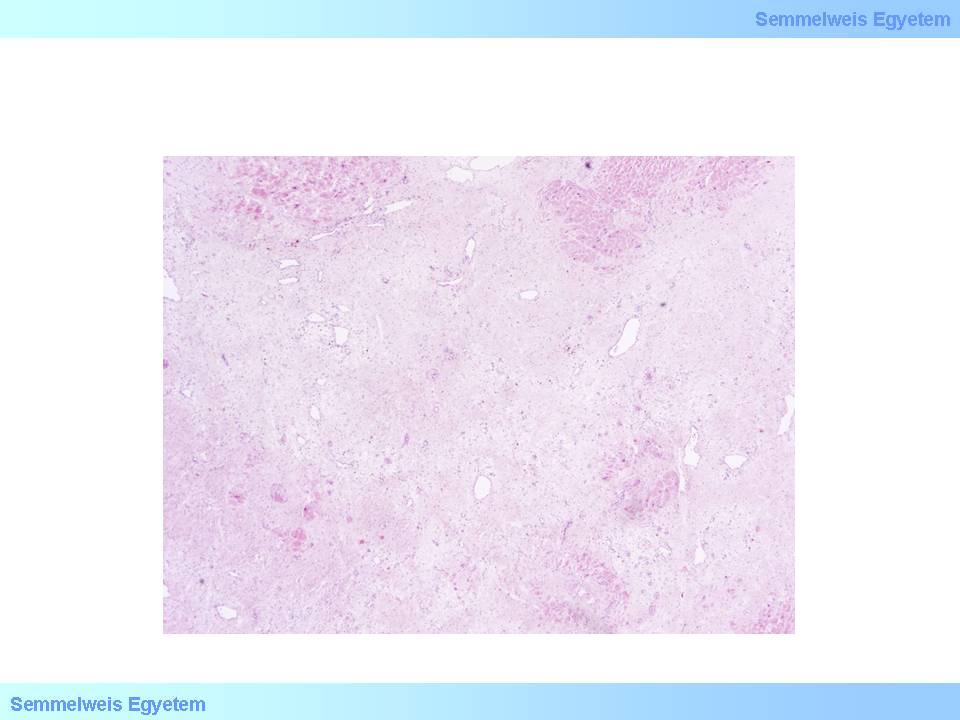
microscopic image No.7: (HE; 100x) Extended scar remaining after myocardial infarction healing.
|
I/2.5.3.5: Paradoxical myocardial infarction
|
 |
An acute myocardial infarction which is not in accordance with the age of the lesion occluding the coronary artery – there is chronic occlusion in the artery, while the supplied area suffers acute infarction. Its prerequisite is to have well functioning collateral circulation between the neighboring myocardial supply areas. With the above circumstances it is possible that after chronic occlusion of a certain coronary artery branch its supply area is supplied by collaterals from a neighbouring area. If an acute stenosis/occlusion (e.g. thrombosis) occurs in the supplying artery of this neighbouring area, it leads to acute infarction in both the supply area of the chronically occluded coronary artery and the neighbouring area.
I/2.5.3.6: Progressive myocardial infarction
An acute myocardial infarction that develops in the marginal zone of a chronic infarction site. It is caused by perifocal (periinfarct) microcirculation disorder and perhaps retrograde growth of the coronary thrombosis in the occluded vessel. The infarct site gradually grows by the formation of more and more infarct zones (so-called wavefront phenomenon).
I/2.5.3.7: Complications of myocardial infarction
I/2.5.3.7.1: Left heart failure and cardiogenic shock
|
 |
Complications involving the insufficiency of the left ventricular pump-function range from mild congestion to the most serious circulatory collapse (cardiogenic shock). First of all, various movement disorders occur in the infarcted myocardium – e.g. hypokinesis (decreased ventricular wall movement) or akinesis (lack of ventricular wall movement). Furthermore, in case of extended infarctions, the remaining working musculature itself might become quantitatively insufficient. However, even without this, significant restriction of the effective heart function might occur e.g. by so-called electromechanical dissociation. Such electric catastrophe might occur in the upper septum even with a tiny infarction focus if it affects an electrical conductive bundle. Independently from the root cause, ventricular fibrillation is very dangerous becase due to unorganized myocardial movements, blood flow practically stops.
If the infarction affects at least 40% of the left ventricular myocardium (macroscopic image No.1P-11) cardiogenic shock occurs. It forces the cardiovascular system into a sort of vicious circle, since decreased circulating blood volume caused by pump insufficiency increases the symphathetic tone which leads to generalized vasoconstriction, therefore the heart’s hemodynamic load increases even more, thereby increasing its insufficiency and fatigue symptoms. The clinical picture might include dyspnea, pulmonary oedema ranging up to cardiac asthma, blood pressure drop, ‘cold sweat’ pallor, acrocyanosis, oliguria, disorientation and loss of consciousness. Cardiogenic shock occurs in 10% of all infarction patients, and it usually complicates the 2nd or 3rd heart attack. Its mortality is dramatically high: 80% with treatment and 90 to 100 percent without treatment.
|

Study the image!
|
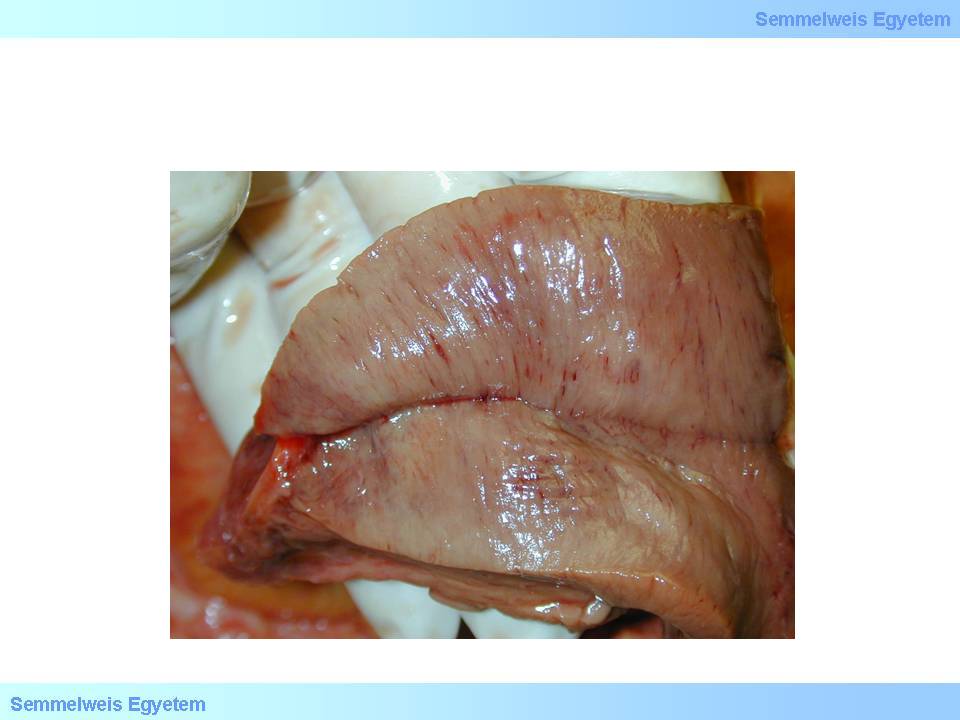
macroscopic image No.11: Subtotal myocardial ischemia. Very rarely – usually due to an extracardial cause – the ischemic insult is such that practically the whole or almost the whole myocardium necrotizes. Such extended loss of the working musculature is by definition not an infarction anymore (but a [sub]total myocardial ischemia), since it doesn’t fulfill the condition that acute ischemic necrosis confines to a vascular supply area. Its clinical manifestation is cardiogenic shock.
|
I/2.5.3.7.2.: Left ventricular aneurysm
Ventricular aneurysm is a bulging of varying extent of some ventricular wall area. It is usually accompanied by wall tapering. According to incidence data, it develops in 15 percent of infarction patients. Pathologically the background of ventricular aneurysm is decreased mechanical resistance of infarcted myocardium and postinfarction scar-tissue which leads to gradual bulging. Acute ventricular aneurysm (macroscopic image No.12) develops in acute infarction stage, during the 1st postinfarction week, along with the destruction of necrotised tissue by macrophages, thereby it often leads to ventricular wall rupture.
|

Study the image carefully!
|
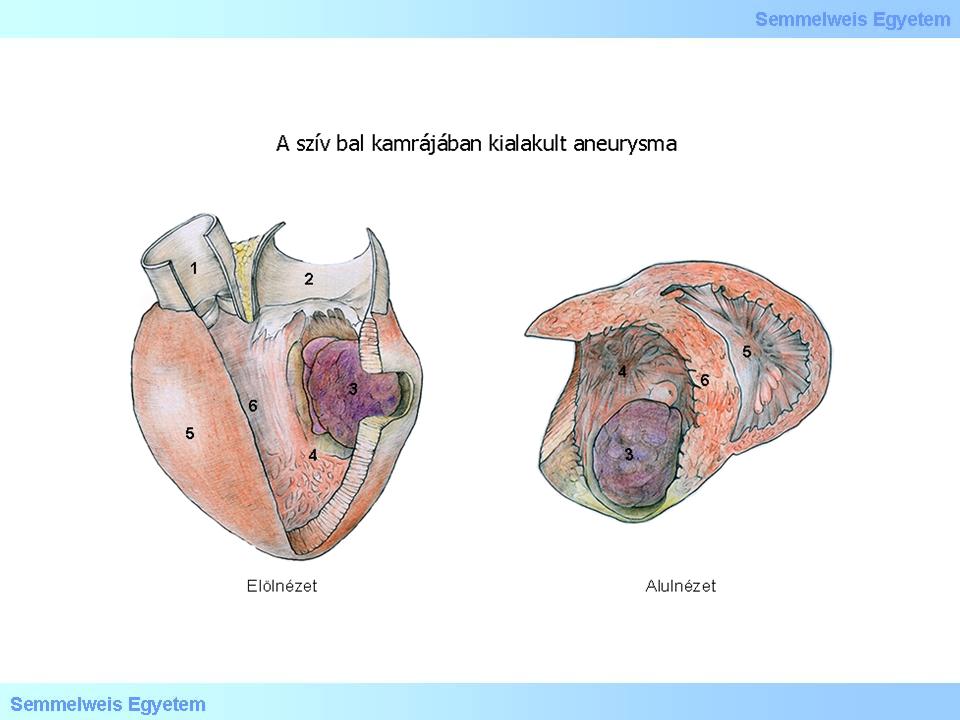
Illustration 1.: (1) Aorta; (2) Atrium sinistrum; (3) Aneurysma; (4) Ventriculus sinister; (5) Ventriculus dexter; (6) Septum interventriculare; (7) Elölnézet; (8) Alulnézet
|
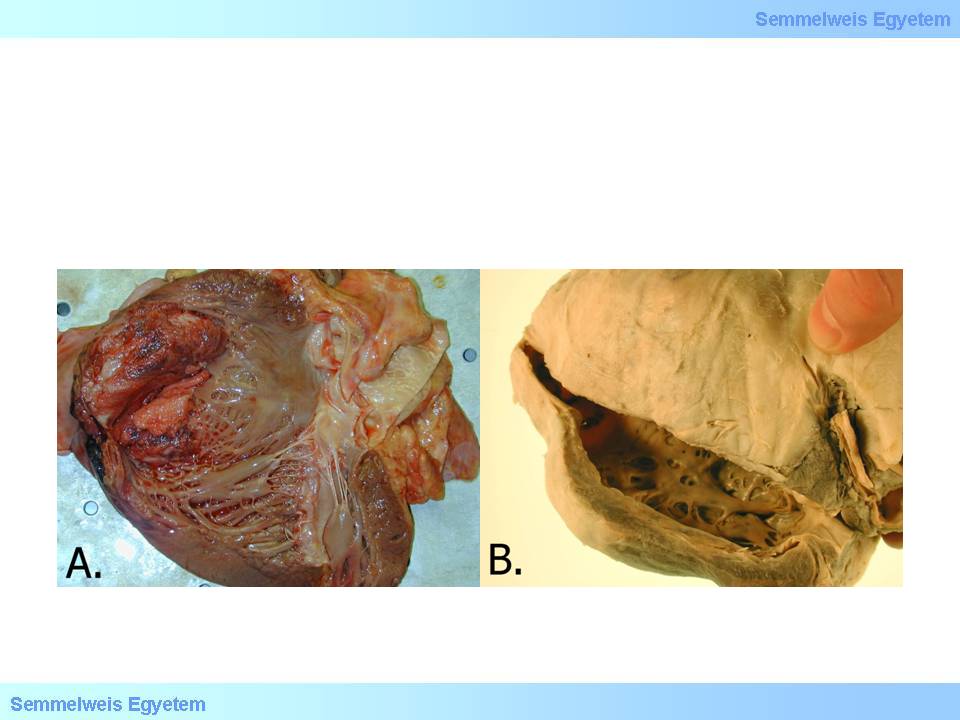
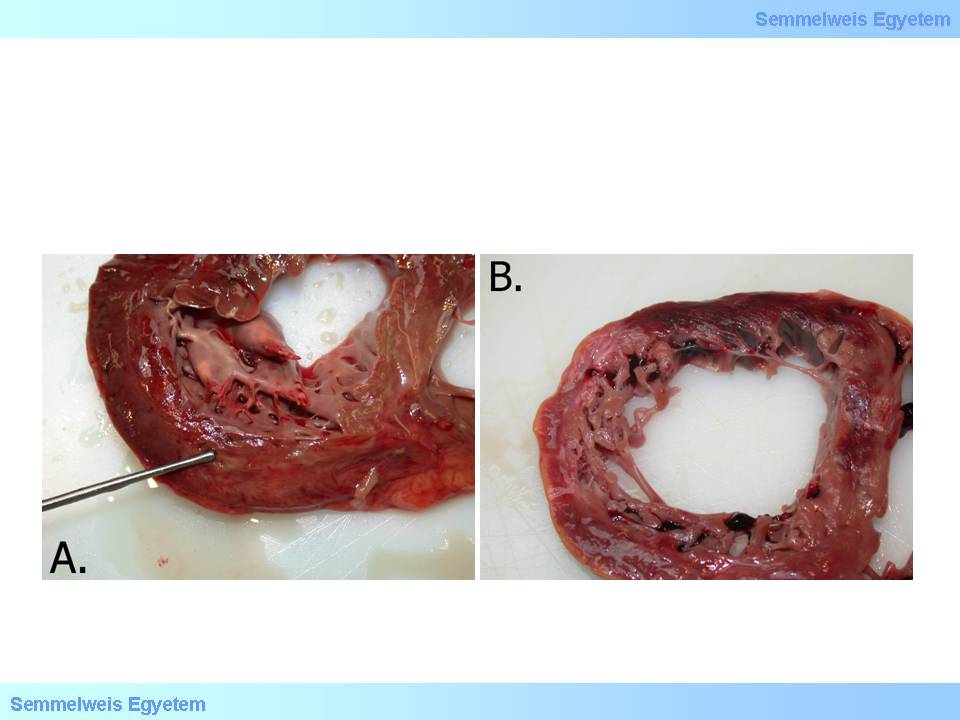
macroscopic images No.12A-B: A: Multitudinous parietal clot development after acute myocardial infarction. Tapering and bulging in the ventricular septum and apex indicates acute left ventricular aneurysm formation. B: Chronic apical left ventricular aneurysm. Note that the wall of the aneurysm is so thin that light can shine through it, and yet it is so resistant due to its persistent connective tissue composition that spontaneous rupture almost never occurs. Its bulging is due to chronic pressure load exerted by the blood content.
|
Chronic ventricular wall aneurysm (macroscopic image No.12B) develops when the infarct site is scarred, months to years after the infarction, due to the scar tissue’s passivity in hemodynamics. Large aneurysms which move to the opposite direction than the intact ventricular wall show so-called paradoxical pulsation . In this case the ventricular blood content moves back and forth between the ventricle and the aneurysm cavity and imposes futile extra work on the already damaged heart muscle. It further decreases coronary circulation in the exhausted and graduallly overloaded heart, and left ventricular failure deteriorates. A common (cca. 50%) risk of aneurysms is thrombosis formation in the aneurysm cavity. Fragments that are torn off from here may cause embolisation in 5% of the cases.
I/2.5.3.7.3: Free ventricular wall rupture, pericardial blood accumulation and tamponade (hemopericardium, pericardial tamponade)
|
 |
It occurs in 10 to 20 percent of lethal infarction cases, most frequently on the 3rd to 5th postinfarction day, as a complication of transmural myocardial infarction. Free ventricular wall rupture leads to rapid pericardial sac filling and the sudden onset fresh pericardial blood accumulation (tamponade – macroscopic image No.13) – due to the pericardium’s unability to expand – obstructs the heart’s diastolic expansion, and practically compresses the heart. Sudden cardiac death is finally caused by a special form of electromechanical dissociation. In this case, the inefficiency of pump-function is not the result of detachment of electrical stimulus generation and conduction from muscle function, but asystolia develops with intact connection between the working musculature and electrical conduction system.
|

Study the image!
|
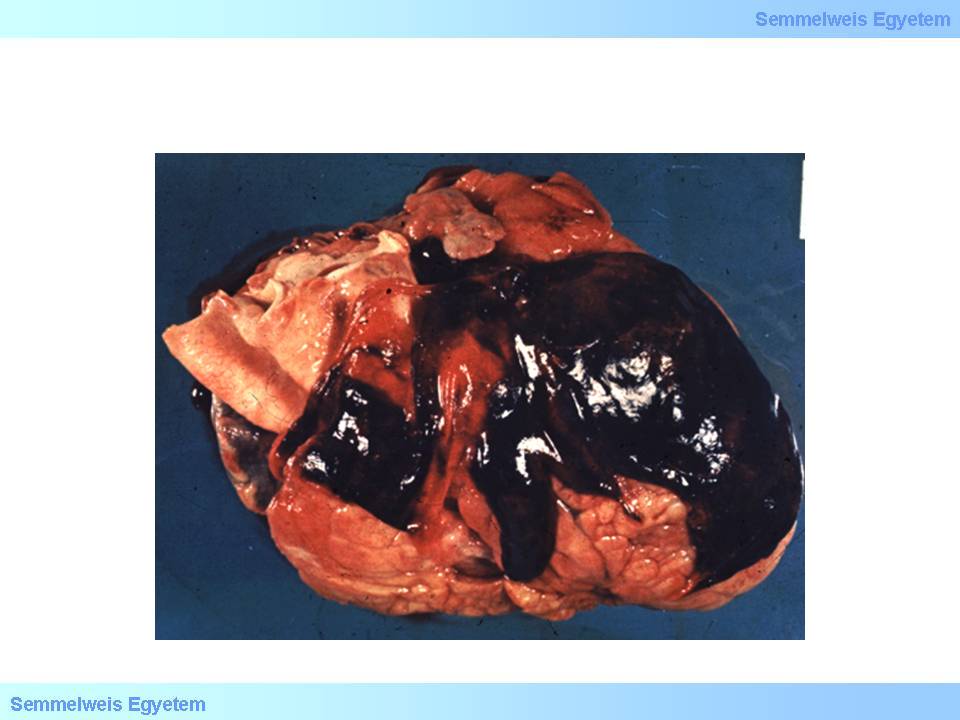
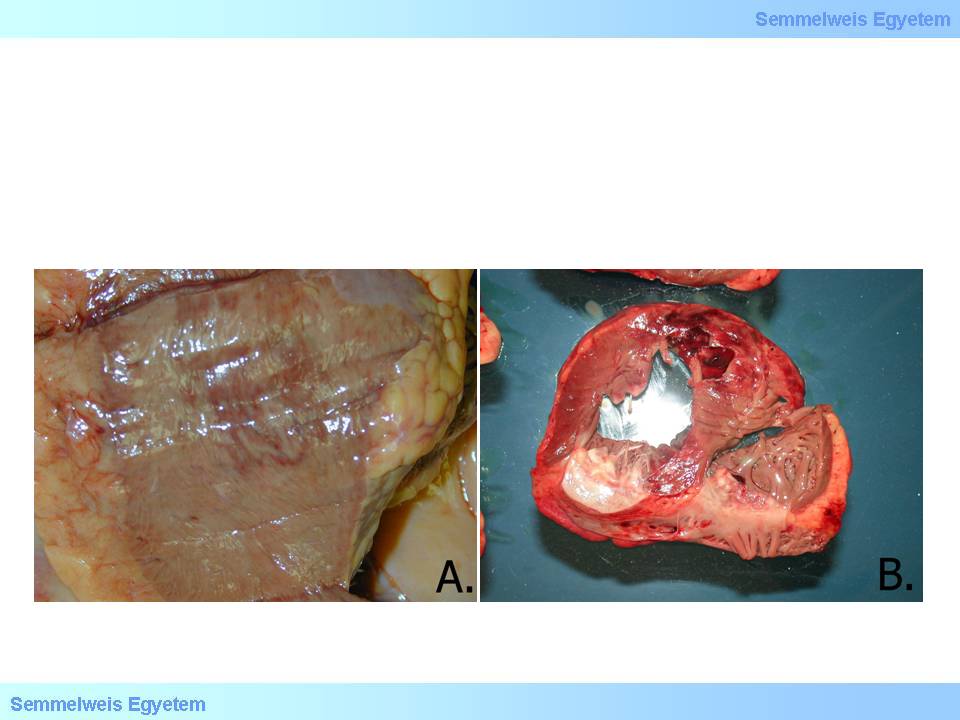
macroscopic image No.13: Pericardial blood accumulation, hemopericardium, cardiac tamponade, pericardial tamponade – these synonyms describe how bleeding breaking into the pericardial sac obstructs heart function.
|
The speed of pericardial fluid filling significantly influeces the patient’s life expectancies. A large rupture with rapid outflow of even small amount of blood (a few ten millilitres) can be lethal, while if the rupture on the wall is not extended and fluid accumulation takes longer time, it leaves time for therapeutic interventions: blood aspiration and surgical intervention. It should be noted that not only blood, but other fluid accumulations can also cause tamponade: exudate in pericarditis, serous transudate which also occurs in the pericardial sac in chronic heart failure, or serosanginous fluid in case of pericardial metastases (carcinomatous pericarditis, pericardial carcinosis). These are typically slow fluid accumulations, and the patients may tolerate even 80-100 mL of that. Factors that predispose for ventricular rupture: transmural infarction; acute ventricular wall aneurysm; old age; female sex; steroid therapy; hypertension; poor collateral circulation; myocardial fibrosis.
|

Study the image!
|
Illustration 3.: Pericarditises exsudatum (izzadmány) - Kiss Balázs
(1) Aorta ascendens; (2) Arcus aortae; (3) Truncus pulmonalis; (4)Vena cava superior; (5) Pericardium; (6) Exsudatum; (7) Arteria interventricularis anterior; (8) Elölnézet.
I/2.5.3.7.4: Ventricular septal rupture
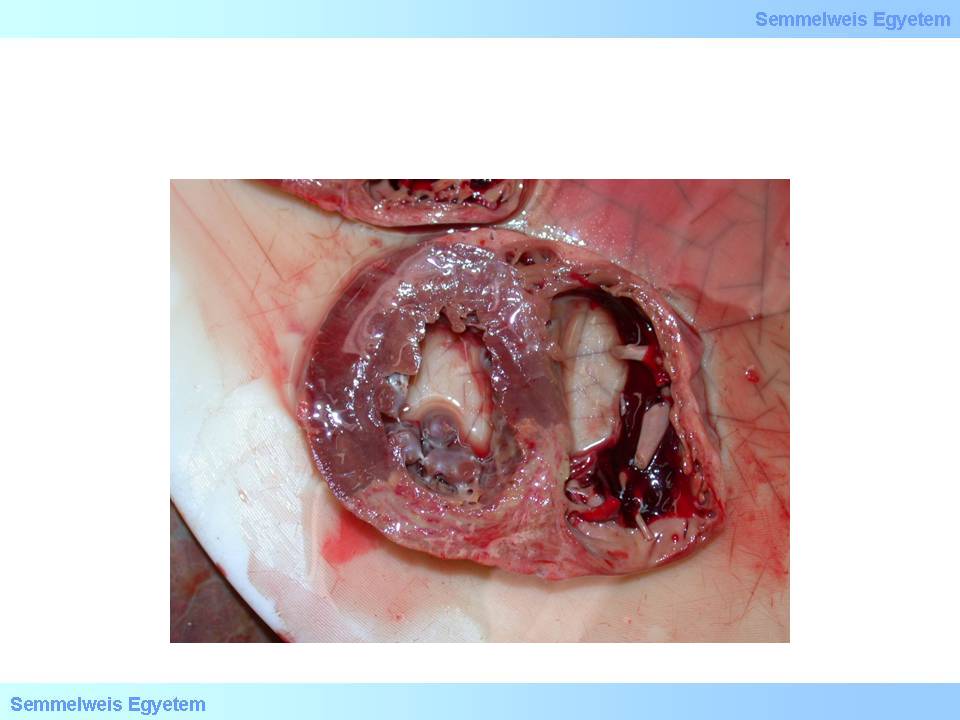
It develops in 1 to 2 percent of all infarctions, typically on the 2nd to 4th postinfarction day. The clinical picture is marked: angina attack, hypotension, acute cardiac failure, or even cardiogenic shock. Hemodynamically, the left ventricular failure is soon followed by right ventricular insufficiency due to left-to-right shunt caused by the septal defect. In more complex cases damage of pump-function may be exacerbated by electronic conductive system lesion too (macroscopic image No.14).
|

Study the image!
|
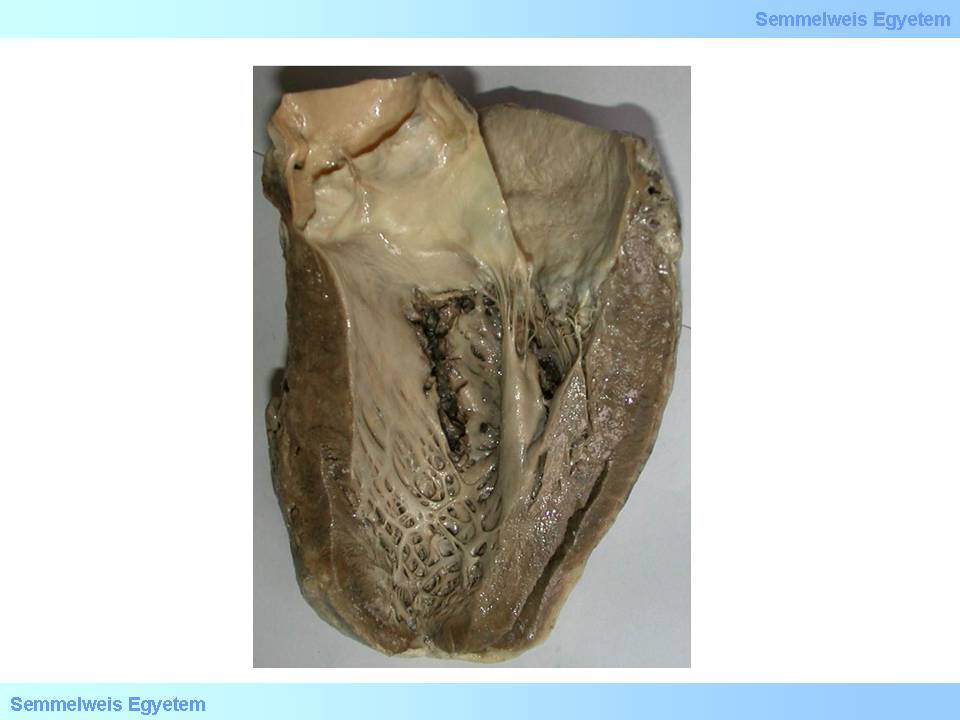
macroscopic image No.14: Septal myocardial rupture. It may lead to sudden left-to-right shunt and electrical conduction disorders.
|
I./2.5.3.7.5: Papillary muscle dysfunction and rupture
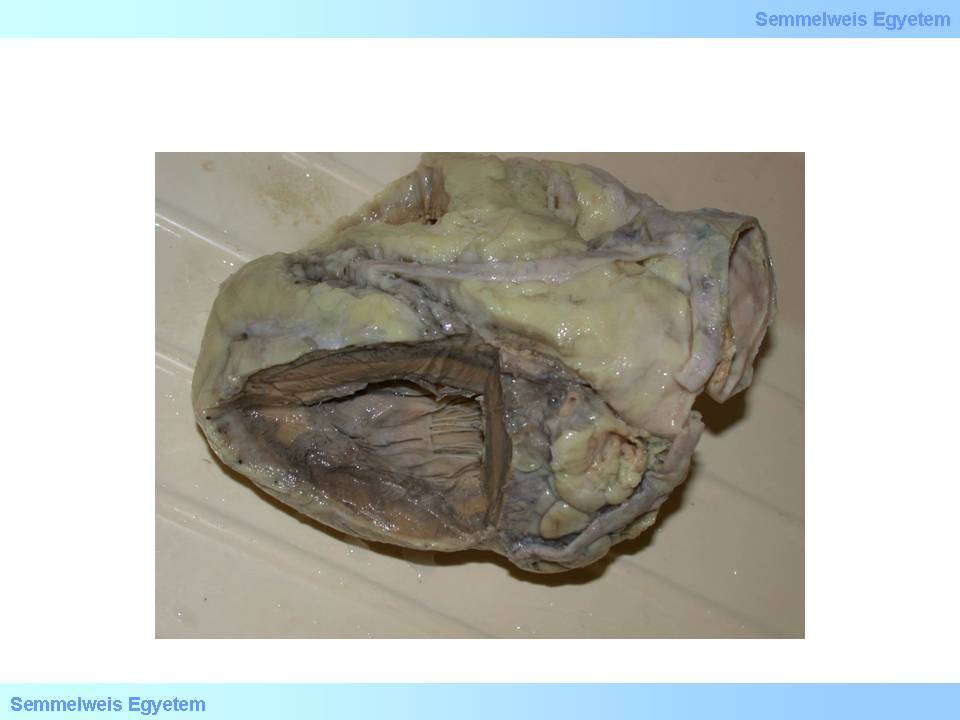
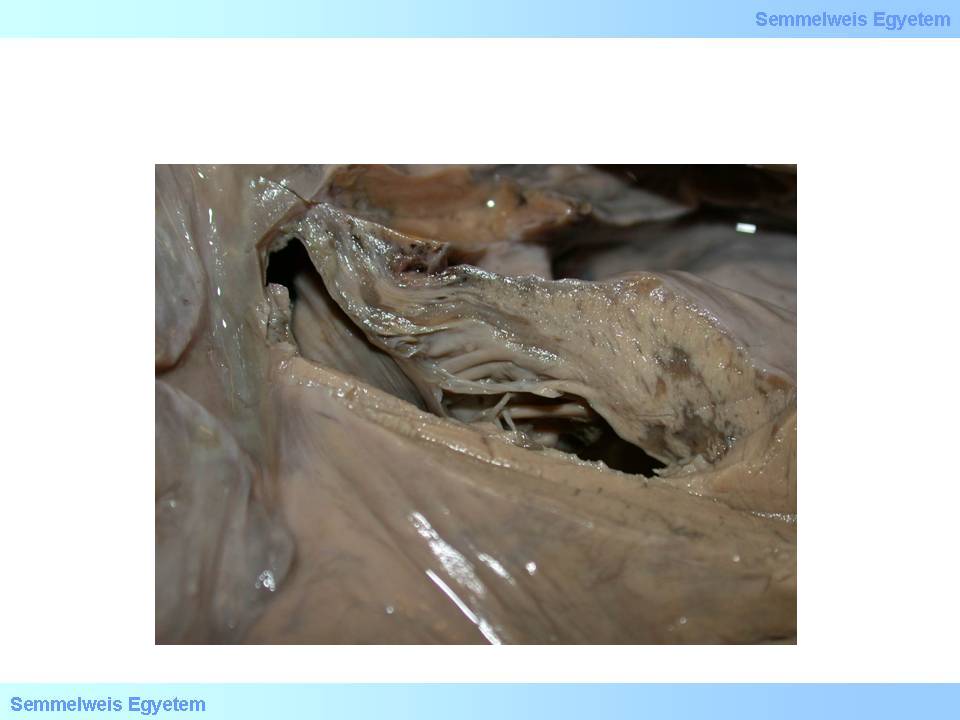
Papillary muscles belong to the subendocardium in metabolic, pathophysiological and pathological aspects, and their function disorder can be detected even during angina pectoris. Definitive papillary muscle damage may occur in myocardial infarction, most frequently on the 2nd to 7th postinfarction day. Rupture of papillary muscles or tendinous cords (macroscopic image No.15) is a life threatening condition: sudden onset severe valvular heart disease (mitral insufficiency) and cardiac failure occurs, and requires urgent artificial valve implantation.
|

Study the image!
|
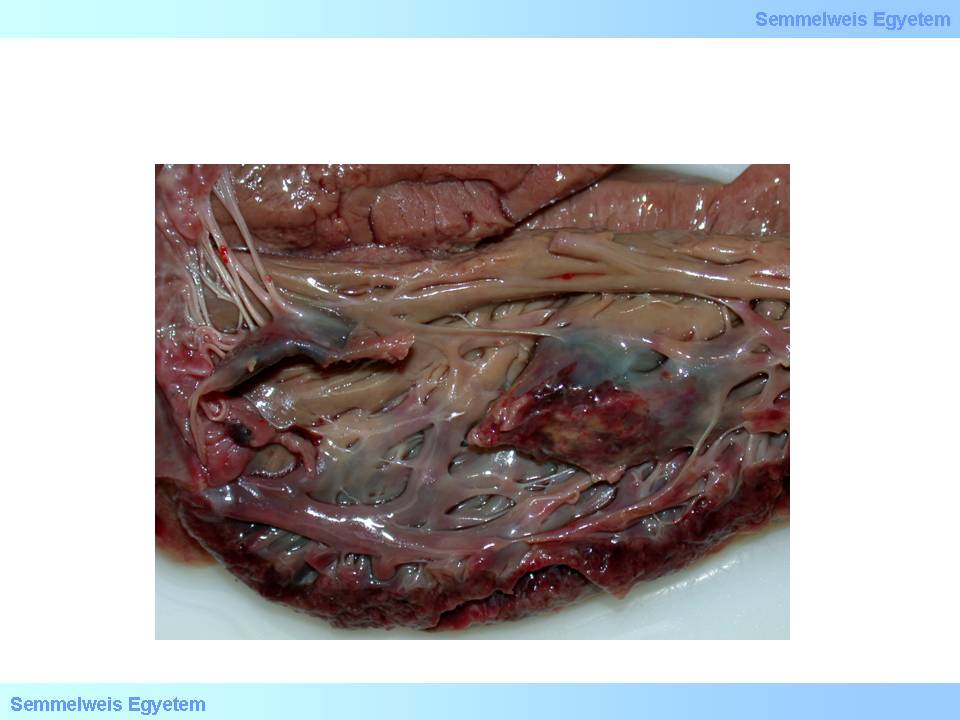
macroscopic image No.15: Papillary muscle rupture: a complication leading to sudden, serious mitral valvular function disorder
|
|

Read the extract!
|
The following interesting extract is the first description of angina pectoris in the world literature and also this publication was the first to use the name angina pectoris.
|
|