| |
VII./2.3.: Meningeoma
VII./2.3.1.: Definition
|
| |
Meningeomas are extraaxial (separating from the parenchyma in most of the cases; extra-parenchymal) tumors, which are originating from specialized meningothelial – creating the arachnoid mater, special „cap”- and trabecular – cells.
Meningothelial cells are covering the arachnoideal villi connecting to the intradural venous sinuses, and their accessory structures.
VII./2.3.2.: Characteristics of the meningothelial cells (with the modification of the listing by Perry et al. 2004.)
VII./2.3.2.1.: Morphological characteristics
|
 |
An important feature of meningothelial cells is, that they create border surfaces by wrapping up around each other, and around the surface of other structures (like vessels, neurons). They are connected by junctions to each other and to the inner surface of the dura mater, they are part of the blood-liquor, and the blood-brain barriers.
The arachnoidal „cap” cells are situated on the outer surface of the arachnoid mater and are covering the arachnoid villi either. The subarachnoid space, filled with cerebro-spinal fluid (CSF), is bridged by fine filaments, known as trabecular arachnoideal cells. Their ultrastructure and epithelial membrane antigen (EMA) positivity refers to their epithelial feature. Many times the fact that they remind to fibroblasts, their capability of synthesizing collagen and other stromal proteins do refer, however, to their mesenchymal feature. Their strong vimentin reactivity is typical. For many pathological effect, they give a fibrosis-like reaction.
VII./2.3.2.2.: Functional characteristics
|
| |
They play an important role in regulating the intracranial (ic) pressure, in maintaining the CSF-homeostasis, as well as in canalizing the CSF, through the valve-like junctions between the liquor space and the blood of the venous sinuses formed by the arachnoid villi. Their secretion activity supports their epithelial features (froming desmosoma and other junctions). They synthetise and excrete numerous liquor proteins (IGF-II, IGFBP-II, apo-E, β2-microglobulin), and -enzymes (prostaglandin D-synthase), glioneural and proliferation factors. Above their tumors, hyperostosis frequently occurs that is generally explained by their alkaline-phosphatase secretion capability. They take part in the excreation of chemokins that are assisting the development of stratum granulosum externum of the cerebellar cortex. They are able to produce gliotrophic, and neurotrophic cytokins either.
VII./2.3.2.3.: Meningothelial cells in pathologic conditions
|
 |
The epitehelial feature of these cells is prominent in the so called secretory meningeomas. In this type of tumor, glandular metaplasia with intracytoplasmic lumen formation is frequent, microvilli, and cilia appear on the surface of the cells. The excreted material is protein-like and known as pseudo-psammoma body. These cells often show cytokeratin and CEA positivity.
Under pathologic conditions their monocyte/macrphag function can manifest too. They can take part in the formation of foreign body-like and rehumatoid type nodules, creation of multinucleated giant cells, in the phagocytosis of lympho-plasmocytoid elements (emperipolesis), as well as in HLA-DR expression.
They may react with hyperplasia to pathologic effects, while extreme damaging effects may even cause their tumorous proliferation. Meningeal proliferation is generally multifocal, it is >10 cellular layers thick, and it develops due to hemorrhage, inflammation, mechanical/chemical irritation, or trauma, in most of the cases. Paradoxically, a tumor might cause it either, at least according to rare, documented cases, for example, a case when a glioblastoma that had grown to the arachnoideal surface caused mechanical irritation and thus, it secondarily induced the development of a sarcomatous meningeoma (sarcoglioma).
The list of functions is not complete, many features of meningeothelial cells are yet unclear. In the recognizing and descripting the special characteristics of these cells, János Kepes, a hungarian neuropathologist, living and working in the USA, played a fundamental role. The colorful functionality scale of these cells explains the high variability of their tumors (meningeomata) too.
VII./2.3.3.: Typical localizations of meningeomas
|
| |
Most of the meningeomas are localized intracranially, or intraspinally. Intracranially, the most frequent locations are on the convexity (Macropicture 1), connected to the falx cerebri (Macropicture 2), in the olfactory groove, along the sphenoid ridge (Macropicture 3), in the region of the sella turcica, in the posterior petrous part of the temporal bone, along the edge of the cerebellar tentorium, in the posterior fossa. The intraventricular plexus choroideus menineomas, originating from the tela choroidea, are rare, and their site predilection in the left ventricule is unexplained.
|

Look into the pictures!
|
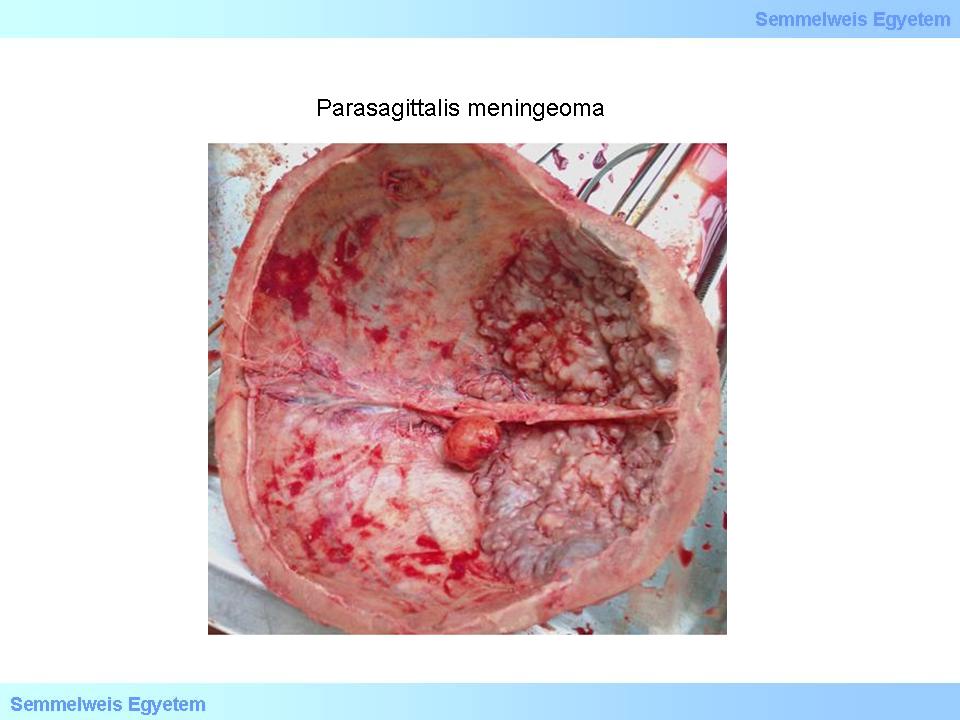
Macropicture 1: Parasagittal meningeoma, that is embedded in the interhemispheric sulcus. It is understandable that these tumors frequently cause headache and epilepsy.
|

Macropicture 2: Parasagittal (falx cerebri) meningeoma. The tumor is indeed tumorous (nodular), with relatively smooth surface. The expressed frontal hyperostosis is independent from the meningeoma.
|
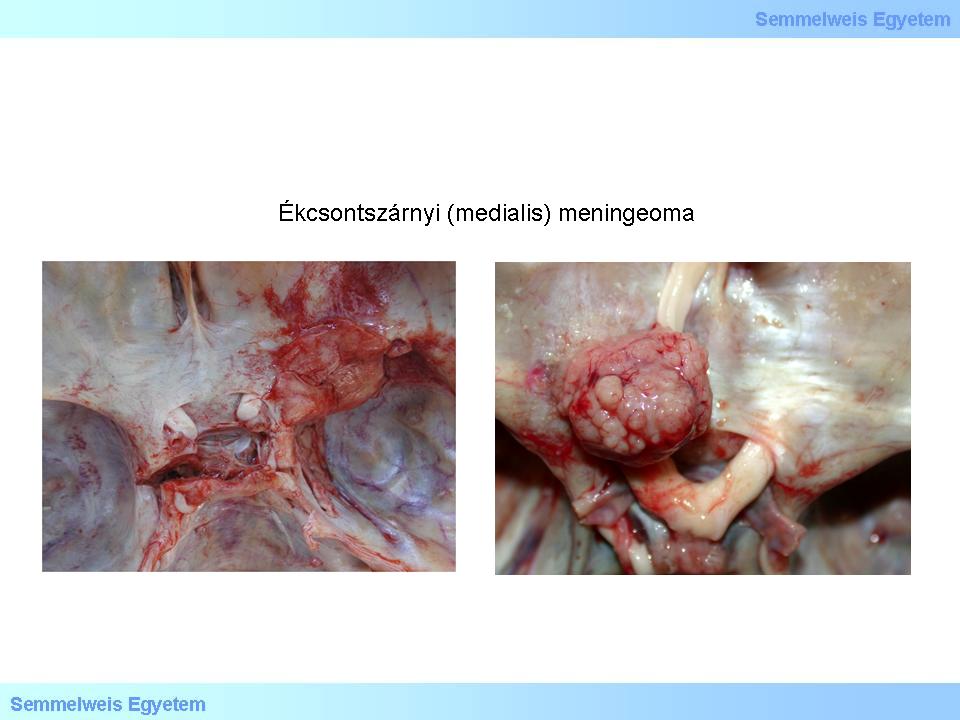
Macropictures 3A-B: Medial meningeoma of the sphenoid ridge. The surface of the tumor is mucous, shiny in some places, while in others it is granular, rough.The removal of the meningeomas of the base of the brain is often impossible due to technical reasons. [20P-Ref5>8P-Ref26]
|
Spinal menigeomas are most frequently located in the thoracic part, meningeomas of the cervical and lumbal parts are rare. Especially rarely, meningeoma may occur outside of the cranio-spinal space, specifically in the head-neck region, for instance, as an orbital (optic nerve sheath), glabellar, sinonasal, oropharyngeal, subgaleal, juxtaparotid, or even cutan meningeoma. In exceptional cases, meningioma may develop farther from the central neuraxis, for example in the mediastinum, in the lungs, in the plexus brachialis.
VII./2.3.4.: Hormonal effects and meningiomas
|
 |
This issue occupies neuro-oncopathologists for a long time. It is known that the female to male gender ratio of the prevalence of meningiomas is 2:1, that can even increase to 9:1 in thoracic/spinal forms. During pregnancy, these tumors often start to grow rapidly, and the compression symptoms of mostly those tumors close to the optic system become more severe, gets better post partum, and in case of another pregnancy, it worsens again. This latter phenomenon is generally explained by the vascular congestion and cytotoxic edema due to pregnancy, since the growth of the tumor mass takes place without the increase in the mitotic activity.
At the same time, in 50-80% of the meningiomas, progesteron-receptors (PR) can be found. The corresponding anti-body shows strong nuclear positivity, while the estrogen-receptor expression is low, and its intensity is weak. The intensity of PR-expression is often inversely proportional to the grade of the tumor, it is not present in anaplastic and malignant meningiomas. Its exact biological role is unclear, however, the increasing risk of meningiomas during hormone therapy and the administration of oral anticoncipients supports hormon-dependency. Additionally, based on the findings of epidemiologic studies, the development of meningiomas are associated with the occurrence of breast cancer, which is a hormon-dependent tumor type either. At the same time, PR-expression is frequently occurring in meningiomas in males and children either.
VII./2.3.5.: Cytogenetic and molecular genetic background of meningiomas
Numerous chromosome abnormality – primarily chromosome loss, leading to hypoploidy – are consistent features of meningiomas. Frequent occurrence of 22 monosomy, partial 22q deletion, as well as loss of chromosome 6, 10, 14, and 18, has to be emphasized. The deletion of 22q can be proved by the FISH method, and LOH- (loss of heterozigosity) studies provide evidence for additional cytogenetic/molecular genetic abnormities accompanying the biological progression of meningiomas through WHO GrI-III. Of course, this consecutive progression of grading is far from linear or necessary at all.
The frequency of chromosome loss supports the existence of meningioma specific tumor-suppressor genes. This is mainly evident in the findings of studies which made the significance of 22q loss clear. NF2 gene is located in 22q12.2 locus, and it is responsible for the synthesis of a protein, called merlin. The second most frequently occurring tumor among those developing due to 22q deletion, is the meningioma (most frequent are the schwannomas). The NF2-associated meningiomas are often multinodal, and it is understandable that they co-occur with type 2 neurofibromatosis (NF-2) many times.
Currently, NF2-gene mutations are thought to be responsible for the development of meningiomas and not for their progression. Sporadic NF2-gene mutation is especially frequent in fibrous and transitional type of meningiomas (see below). Interestingly, it can be found only in 25% of meningiomas, and specifically in those developing on the base of skull. This supports the existence of alternative, non-NF2 associated meningioma-induction pathways. In case of finding meningioma in children or in adolescents, the possibility of latent NF-2 has to be considered, and diagnostic tests have to be carried out. Majority of the genes responsible for the biological progression of meningiomas, are yet unknown. Here,it is enough to mention that the deletion of the CDKN2A/p16 region (9p21 locus) indicates a worse prognosis for anaplastic meningiomas. Thus, 9p21 FISH tests may have prognostic value!
VII./2.3.6.: Clinicopathologic background, biologic potential
|
 |
And thus, these tumors typically develop from the meninges, grow slowly, are firmly attached to the dura mater, are constituted of arachnoideal cells, mainly develop in adults, but sometimes they can occur in children and adolescents too, and are dominantly occurring in females. Ionizing radiation of the skull have been proved to be an important risk factor by numerous, independent epidemiologic studies. Those tumors associated with irradiation, are mainly multinodal, more frequently show histologic atypia, and are more clinically agressive. Both benign and malignant forms exist. Neuroradiologically, typical meningiomas are solid, round or lobular tumor masses, connected widely to the dura.
Rarely, the tumor body is not node-like, but covers the originating site as a flat layer: meningeoma en plaque. Primarily, it grows by pushing the adjacent structures aside, however, infiltrating the dura, or the nearby venous sinuses is not rare either. It may also occur that the tumor infiltrates/breaks through the bones and it can be palpated on the outer surface, as a subaponeurotic-subcutan tumescence. These macroscopic features do not necessary indicate negative biological potential (like malignity).
With respect to the clinical course, recurring tendency is especially significant. The recurrence of the various types of meningiomas are highly dependent on the radicality of surgical removement, that is fundamentally determined by the anatomical localization (Macropicture 20P-3). It is important to know, that even in cases when surgical removement is judged as total (in toto), recurrence is not rare, and the molecular background of the process of recurrence is unknown. The infiltration of dura mater can be observed in almost all cases (Macropicture 4). Despite that the infiltration, or invasion of cerebral parenchyma per se does not indicate malignity, according to the most novel oppinion, these tumors („brain invasive meningeoma”) have to be classified as WHO GrII, regardless their histological characteristics, and patients have to be followed with extreme care, since this phenomenon is a sign of agressive clincal behavior.
|

Look into the pictures!
|
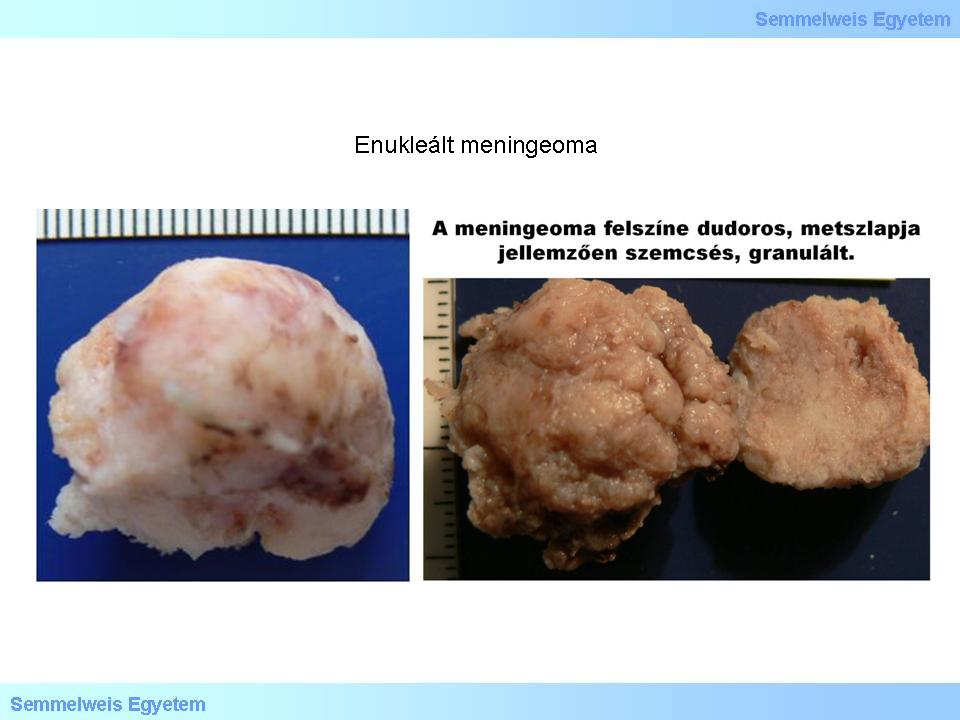
Macropictures 4A-B: Enucleated meningeoma. The tumor shows a tubercular shape, with rough edges, its surface is tuberous, but smooth at the same time (a seeming inconsisteny), the cut-surface is typically granulous. Hemorrhages can be seen as brownish red areas.
|
Majority of the meningiomas are isodens with the cortex in CT and T1-weighted MR images, which makes it difficult to determine their borders. After applying a contrast enhancement material, the „dural tail”-sign can be observed, which is a reliable sign of the tumor being indeed extraaxial. Neuroradiological signs of higher grade tumors are the T1 and T2 hyperintensity, blurred edges of the tumor mass, the so called „mushrooming” (multinodular masses grow into the cerebral parenchyma), in T1 images, dark, in T2 images, light spots within the tumor (tumor-necroses).
VII./2.3.7.: Macroscopic appearance
The shape of the tumors is variable, it is often nodular, almost potato-like, but there are forms which are connected to the dura mater as thin layers (see also the so called en plaque tumors). Predominantly, these tumors are well-demarcated, compact, often show rubber-like elasticity, however, not rarely they are hard, show sand-like granulosis („grittyness”), that refer to either calcification, or to the massive presence of psammomabodies (Macropicture 5). It is understandable, that it is difficult to create either smear/frozen section, or paraffin embedded preparation from this tumor. Necrotic and hemorrhage nodules are softer, rarely they give also a gelatinous feel, which is generally caused by mucous degeneration.
|

Look into the pictures!
|
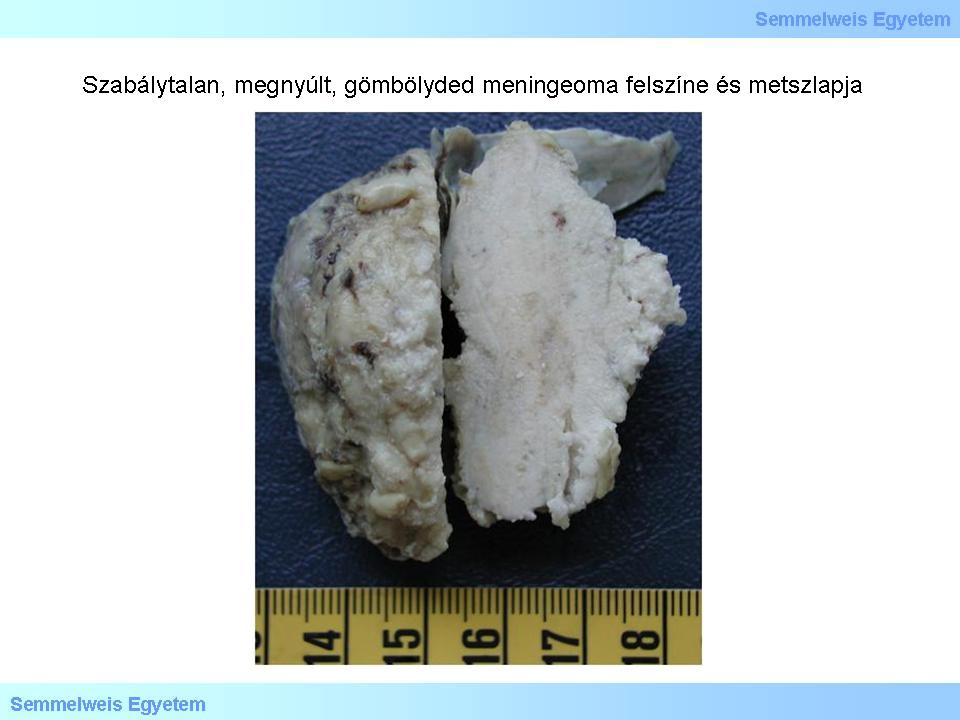
Macropicture 5: Surface and cut-surface of an irregular, elongated, round meningeoma. The surface is micronodular, the cut-surface is slightly rough, „gritty”, generally denoting the massive presence of psammomabodies. The part of the dura mater connected to the tumor is well observable, this is called as the „dural tail”, on neuroradiologic images.
|
Their cut-surface is generally homogeneous, but it is also not rarely fasciculated, whirling (similarly to leiomyomas Myoma uteri]). The dominant color is greyish-white, but blackish-red (hemorrhage), and yellow (increased lipid content: xanthomatous transformation, metaplasia of adipose tissue) spots can also occur. The nodules of bone/chondroid metaplasia are white. Tiny hemorrhages may occur in the adjacent, compressed cerebral tissue.
The „classic” type of meningeomas (see below) frequently develop below the frontal lobe, in the olfactory region (subfrontal meningeoma). Sometimes this tumor grows astonishingly big, especially considering the available space, and pushes upwards the frontal lobes, sometimes the hypothalamus too. Significant space occupations may suppress the ventricular system either. Gradual deterioration, as well as substantial changes in the behavior, thinking process, intellectual capacity of the patients, is relatively common in these cases. It is typical that behavior turns agressive, the patient becomes hostile with the environment, and tantrums and obscene verbality are taking turns. Because of these symptoms, patients may need psychiatric treatment. If the underlying cause of these clinical symptoms is not diagnosed during psychiatric treatment, it is often only the autopsy that reveals the meningioma in the background of the frontal lobe dysfunction, instead of dementia.
VII./2.3.8.: Pathohistology of meningiomas
VII./2.3.8.1.: General characteristics
|
 |
Most frequent are the grade I tumors, which are histologically and biologically benign, but some of their histological subtypes are graded as GRII or III, because their prognosis and clinical course are poorer. Recurring tendency and increased agressivity is characteristic when the proliferation index is high, and/or the tumor infiltrates the brain tissue. The histopathological picture is not always decisive with regard to the prognosis. Immune-histochemical determination of the proliferation activity is a useful tool, from which the immune-histochemical reaction with Ki67 (MIB-1) monocloncal antibody became widely used.
It has to be noted, however, that the association between the Mib-1 (Ki-67) immune-histochemical labelling index (LI) and biological behavior is much less reliable compared with the de facto counting of the number of mitotic activity in HE preparations. If in any meningiomas, >20 mitosis/10 HPF (high power field; the field is examined with a 40x objective, meaning cca. 400x magnification) can be counted, that tumor is considered malignant, regardless of its level of differentiation. It is still subject of debate whether the Mib-1 LI per se is an independent prognostic feature, especially, because the subjective evaluation of the labeling intensity of the IHC methods varies across laboratories.
VII./2.3.8.2.: Histopathological grading
The current WHO classification (2007) differentiates16 types/variants, all of which can be classified into 3 grades (Table 1).
|

Look into the classification!
|
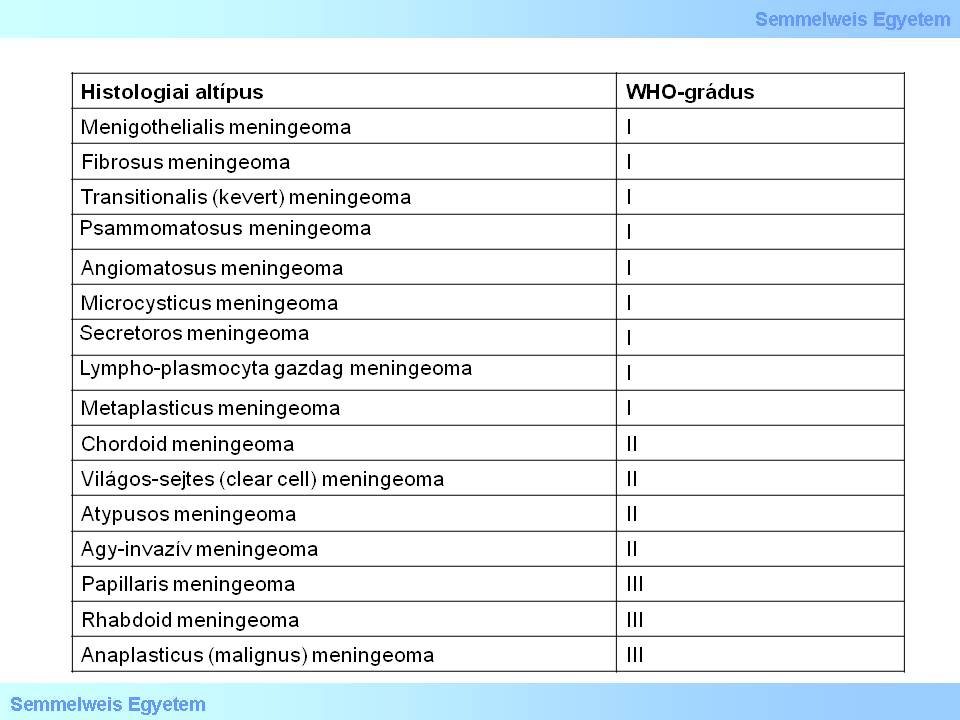
Table 1.
|
VII./2.3.8.3.: „Classic” histologic types
One of the most frequent „classic” hyistologic type is the meningothelial meningeoma. It is typical in the micropicture that arachnoideal cells form nests loosely fitting to each other, as an irregular mosaic. The appearance of the cells is rather monomorphic, nuclei are typical. Regressive lipidisation (xanthomatous transformation) or mucous degeneration is not rare in the central part of the tumors. The presence of small, light, intranuclear inclusion (pseudoinclusio), sometimes in a great number, in the nuclei, is characteristic for meningothelial meningiomas, and may also be useful in the differential diganosis. The so called meningothelial lobules surrounded by fibrous septums are also a characteristic feature of this type of meningioma. Sporadically, psammoma bodies may also occur, but the number of psammoma bodies in meningothelial meningiomas is typically small. Nonetheless, there is a menigioma type showing a great number of psammoma bodies, the so called psammomatous type.
The other „classic” meningioma type is the fibrous meningioma (some use the attribute „fibroblastoid” but it is incorrect, since these cells are not fibroblasts, but meningothelial elements). Fasciculi of spindle- and cigar-like cells (indeed similar to fibroblasts, by the way), with central, hyperchrome nuclei, ordering side by side are typical for the histological appearance. These fasciculi sometimes cross each other, form networks, and generally there is a mass of collagen and reticulin fibers among them, the quantity of which can be pronounced.
VII./2.3.8.4.: Other histological types
|
 |
The transitional meningioma is a mixture of the meningothelial and fibrous types. The microcystic meningeoma is characterised by the numerous, slim tumor cell extensions, causing a pitted network image of the tumor. The lymphoplasmocyte-rich meningioma may contain chronic inflammatory cells in such a great number that it can be a differential diagnostic challenge to differentiate it from a chronic inflammatory process. The chordoid meningioma, as it is already suggested by the name, in its appearance, is similar to notochord originated chordomas. The metaplastic meningioma may contain bone, chondroid, or adipose tissue. The lipomatous meningioma is most probably characterised by progressive xanthomatous degeneration and non-specific lipidasition instead of real metaplasia.
A sclerotizing meningioma is a type not included in the WHO classification, however it occurs. It is characterized by the mass of so called amanthioid collagen, which shows hyalinoid degeneration. These thick fibers often form round structures, even with a diameter of few hundred µm. The oncocytic meningioma and the myxoid/mucinous meningioma are also missing from the WHO classification. The latter overlaps with the chordoid and myxoid-metaplastic categories. The clear-cell meningioma,the rhabdoid meningioma (Micropicture 1) and the papillar meningioma are higher grade types (see a0lso below). Criteria of the atypic meningioma has been first clarified by Arie Perry et al, that was taken over by the WHO in 2000, and then partly modified in 2007. The anaplastic meningioma (meningioma malignum) means tumors with fibrosarcoma-like, anaplastic carcinoma mimicing appearance, and melanoma-like structure.
|

Analyze the tissue on the picture!
|

Micropicture 1: Rhabdoid meningeoma. It is per definitionem a high grade histologic type.
|
VII./2.3.8.5.: Grading criteria according to the WHO
This is one of the most debated fields of neuro-oncopathology. WHO classifies four subtypes per definitionem in higher grade:
-
1. the „clear cell” meningioma (Gr. II.),
-
2. the chordoid meningioma (Gr. II.),
-
3. the rhabdoid meningioma (Gr. III.), and
-
4. the papillar meningioma (Gr. III.).
It is of debate still today, that for indicating the higher grade, how extended the affected area should be.
VII./2.3.8.5.1.: Benign meningioma (WHO GrI.)
Any predominant histological picture, except for chordoid-, clear cell-, papillar-, or rhabdoid pattern/cell type. Criteria of atypic-, brain invasive-, and anaplastic meningiomas are missing.
VII./2.3.8.5.2.: Atypic meningioma (WHO GrII.)
(Micropicture 20P-2). Increased rate of mitotic activity:: ≥4 mitosis/10 HPF. Important: it is not Ki-67 IHC result! Additionally, at least 3 of the 5 parameters below have to be present:
-
1. „sheeting” – without structure, a field like growing pattern, without whirls, nests, septums, lobules, etc.;
-
2. Microcytic transformation, namely, lymphocyte-like tumor cells with high nuclear-cytoplasmic ratio (N/C ratio);
-
3. hypercellularity;
-
4. prominent nucleoli: so called „macronucleoli”;
-
5. spontaneous necrosis (not due to embolisation or irradiation; but sui generis tumornecrosis).
|

Analyze the tissue on the picture!
|

Micropicture 2: Atypic meningeoma. Characterized by the lack of pattern (sheeting), and pleomorphism.
|
VII./2.3.8.5.3. Brain-invasive meningioma (WHO GrII.)
The tumorous meningeal tissue invades the cerebral tissue in the form of tongue-like protrusions. The relatively sharply demarcated feature of these protrusions might be misleading. In the substance of the meningioma GFAP-positive neuropil-islands can be detected („incorporation”).
VII./2.3.8.5.4.: Anaplastic (malignant) meningioma (WHO GrIII.)
At least one of the two criteria below has to be present:
-
- mitotic index ≥ 20 mitosis/10 HPF.
-
- definite anaplasia (sarcoma-, carcinoma- or melanoma like cytology/histology).
The classification above uses numerous subjective criteria (e.g. „sheeting”, presence of microcells, etc.), however, this is the accepted system which is applied worldwide. It can be expected that the upcoming WHO classification will take into consideration much more molecular/genetic viewpoints.
|
|