| |
I/2.4: Hepatic tumors
I/2.4.1: Classification of focal hepatic lesions
|
 |
1: Primary hepatic tumors
Benign epithelial tumors
-
– hepatocellular adenoma
-
– cholangiocellular adenoma
Malignant epithelial tumors
-
– hepatocellular carcinoma
-
– cholangiocellular carcinoma
-
– mixed cholangio-hepatocellular carcinoma
-
– hepatoblastoma
Benign non-epithelial tumors
-
– cavernous hemangioma
-
– fibroma
-
– angiolipoma
Malignant non-epithelial tumors
-
– hemangiosarcoma
-
– hemangioendotheloma
-
– embrional sarcoma
-
– lymphoma
2: Secondary (metastatic) hepatic tumors
3: Tumor-like focal hepatic lesions
-
– focal nodular hyperplasia
-
– nodular regenerative hyperplasia
-
– mesenchymal hamartoma
-
– cysts
-
– Echinococcus-cyst (macroscopic image No.1)
-
– biliary cyst
-
– peritoneal (so-called inclusion) cyst
-
– von Meyenburg-komplex
-
– inflammatoric pseudotumor
-
– hepatic abscess
-
– infarction
-
– peliosis hepatis
|

Study the photo!
|

macroscopic image No.1: Echinococcus-cyst. Humans are intermediate hosts in the life cycle of the parasite, and its cyst is surrounded by a connective tissue capsule. If the cyst breaks it may lead to anaphylactic shock. (From the collection of the 2nd Institute for Pathology, Semmelweis University – collected by Attila Kovács and Ildikó Szirtes).
|
I/2.4.2: Hepatocellular adenoma
|
 |
A benign tumor originating from hepatocytes, most frequently in young women. This tumor is suggested to be related to oral contraceptive and androgenic steroid use. Hepatocellular adenoma is macroscopically single, regular sphere shaped lesion of variable size, sharp outline and surrounded by a capsule, and its parenchyma is similar to that of the liver: homogenous with brownish-yellowish of brownish-reddish colour. Hemorrhages and necroses are often seen.
Histologically, it consists of polygonal tumor cells that resemble hepatocytes. The tumor tissue sometimes contains several irregular vessels, and always lacks portal areas and biliary ducts. Those adenomas that grow under the hepatic capsule may lead to tumor rupture, bleeding, and so-called acute abdominal syndrome. In this case, other diseases that can lead to acute abdominal syndrome (e.g. tubal pregnancy in female patients) should be considered for differential diagnosis.
I/2.4.3: Cholangiocellular adenoma
A benign tumor originating from the bile duct epithel. Macroscopically the tumor is either single or multiple, greyish-white, compact, and is usually <1 cm. It is often located under the hepatic capsule. Histologically abnormal, biliary duct-like structures are seen, which are lined by typical cuboidal or columnar epithelium resembling the epithelium of biliary ducts, and are surrounded by thick connective tissue stroma rich in collagenic fibres. For differential diagnosis – especially in case of multifocal, subcapsular cholangiocellular adenoma – carcinoma metastases (e.g. from pancreatic carcinoma), primary cholangiocellular carcinoma, or biliary hamartoma (von Meyenburg complex) should be considered.
I/2.4.4: Hepatocellular carcinoma (HCC)
1: Epidemiology, patogenesis
|
 |
The most frequent primary malignant tumor of the liver, hepatocellular carcinoma is the fifth most frequent malignant tumor in the world, and takes the third place in the tumor related death statistics, with significant geographical variations. Its development is influenced by several factors. Its causal factors include hepatotropic virus infections (HCV, HBV), alcohol, aflatoxin (grain fungus), some drugs (e.g. androgenic steroids); incidence increases in some rare hereditary metabolic diseases such as certain types of glycogen storage diseases, hemochromatosis, alpha-1-antitrypsin deficiency, tyrosinemia, hypercitrullinemia, porphyria cutanea tarda, hereditary hemochromatosis (its most common mutation is C287Y), Wilson’s disease, and glycogenosis.
Hepatic cancer is very frequent at certain geographical areas (e.g. Africa, Far East) due to endemic HBV infections and the viral genom can be detected in both the cancer cells and the not carcinomatous liver cells. It was verified that some viral products (e.g. the protein product of the HBV’s so-called X-gene) promote malignant transformation. In most of the cases (70-80%) hepatocellular carcinoma is preceded by cirrhosis: in the vast majority of these cases the antecedent is hepatocyte necrosis associated damage and proliferation that aims regeneration during this constant cell-death, but original status can’t be restored due to severe structural distorsions, and the chance of genetic errors due to internal or external causes increases.
First hyperplastic, then dysplastic foci appear, and finally develops hepatocellular carcinoma as the malignant phenotype. Genetic alterations can be detected in relation with tumor development, such as p53 and other suppressor gene mutations, and increased expression of various growth factors. The serological and immunohistochemical marker of HCC is alpha-fetoprotein (AFP) which is an important parameter for follow-up both at the first diagnosis and in case of cancer recurrence. (AFP is a physiological tissue marker in the fetal and neonatal period, but later it is not expressed anymore, and its reappearance indicates tumor development. Other tumors may also be AFP+, e.g. germ cell tumors or pancreatoblastoma).
2: Morphology
There are various macroscopical forms of HCC.
-
- The so-called massive form is a solitary (larger) tumor nodule surrounded by a pseudocapsule
-
- In the nodular form there are tumor nodules of various sizes
-
- In cirrhosis carcinomatosa there are several small tumor foci that fit the pseudo-lobules in size; this is a relatively rare form.
The often macroscopically obvious development of satellite tumors (spreaded tumors) is also typical (macroscopical image No.2), as well as invasion into the venous system. The color of the tumor is usually brownish-green, well differentiated tumors are able to produce bile, therefore their color is deep green or greenish (macroscopical image No.3), or more rarely greyish-whitish-red (macroscopical image No.4). Extended tumor necrosis may occur, and it is macroscopically detected as softening.
|

Study the images, and analyze what you see!
|
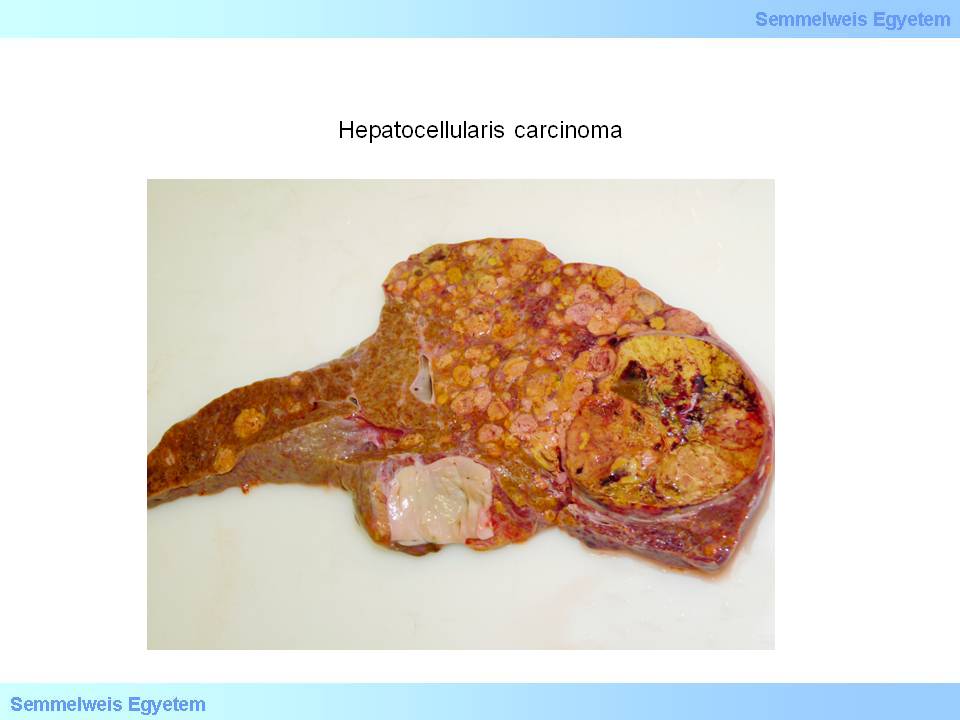
Caption: macroscopical image No.2: Hepatocellular carcinoma. Satellite tumors appear around a solitary tumor nodule. (From the collection of the 2nd Institute for Pathology, Semmelweis University – collected by Attila Kovács and Ildikó Szirtes).
|
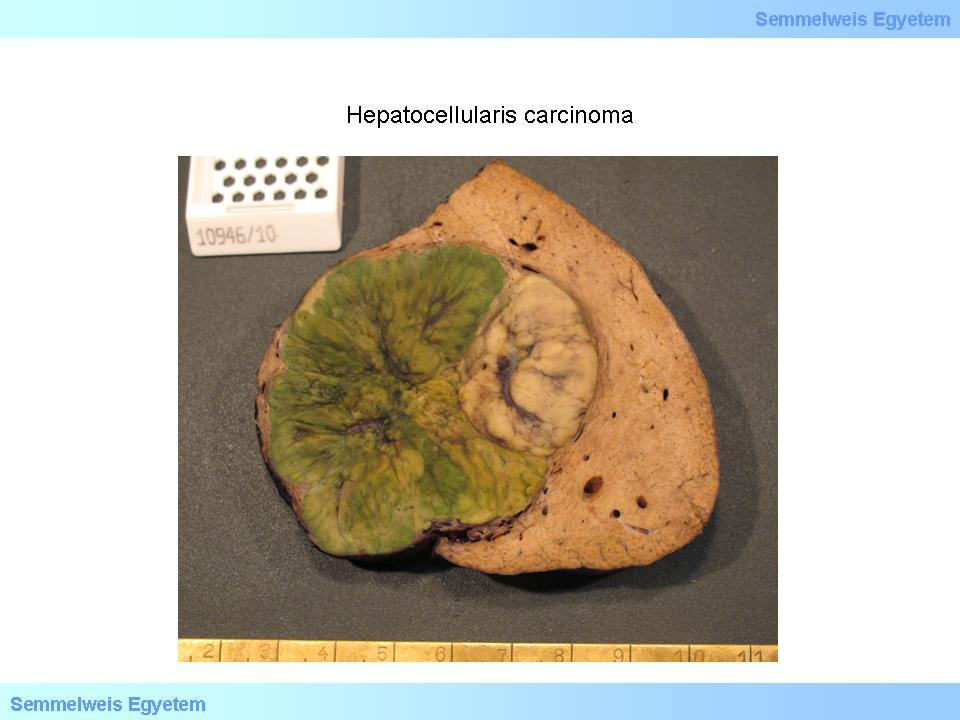
Caption: macroscopical image No.3: Hepatocellular carcinoma. The characteristic green color of well differentiated tumors is due to intact bile production of the tumor cells. (From the collection of the 2nd Institute for Pathology, Semmelweis University – collected by Attila Kovács and Ildikó Szirtes).
|
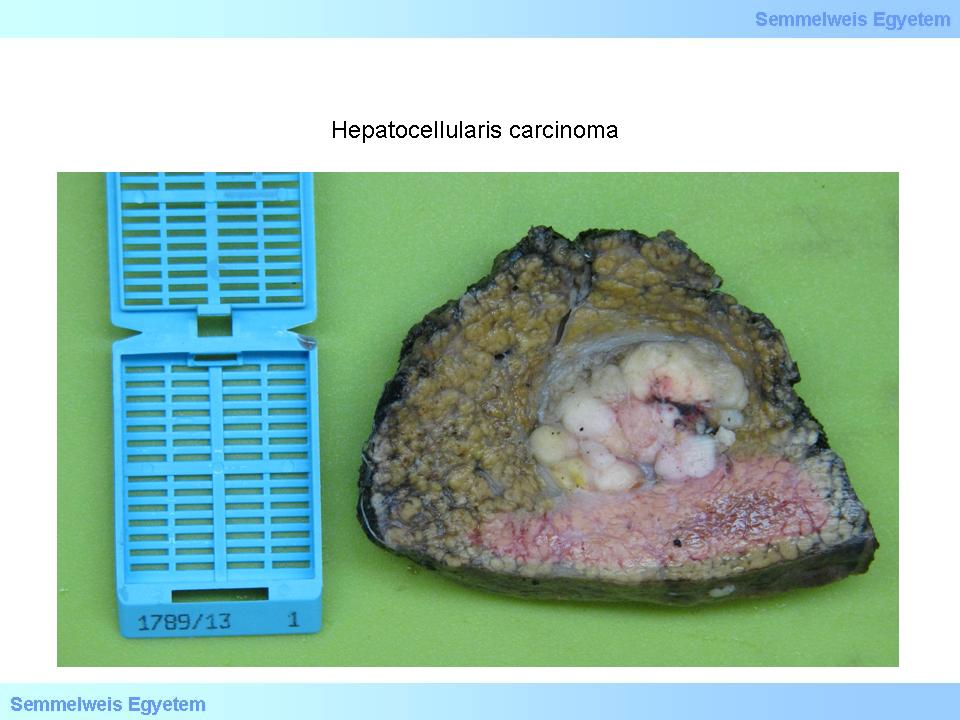
Caption: macroscopical image No.4: Hepatocellular carcinoma. Sometimes the tumors are greyish-white which is also characteristic of metastatic foci. Cirrhotic environment suggests primary tumor here, since – according to pathological experience – metastases rarely occur in cirrhotic livers. This can probably be explained by portal hypertension, because impeded portal inflow may prevent tumor cells from getting into the liver. (From the collection of the 2nd Institute for Pathology, Semmelweis University – collected by Attila Kovács and Ildikó Szirtes).
|
The following forms can be distinguished acccording to the histological structure:
(i) trabecular,
(ii) tubular,
(iii) alveolar, and
(iv) special forms (clear cell, pleomorphic-anaplastic, fibrous).
According to the degree of differentiation (grading) we distinguish well differentiated tumors (grade I) that are difficult to tell apart from hepatic tissue or adenoma; moderately and mildly differentiated (grade II-III) tumors where some of the tissue structures are still discernible (microscopic images No.3 and 4); and undifferentiated (grade IV) tumors where there are severe cytopathological alterations, and the tumor’s origin can only be verified by specific tests. ’Nodule in nodule’ pattern indicates an agressive (high-grade) transformation. Hepatic cancer cells often show alpha-1-antitrypsin or alpha fetoprotein (AFP) positivity.
|

Study the images, and analyze what you see!
|
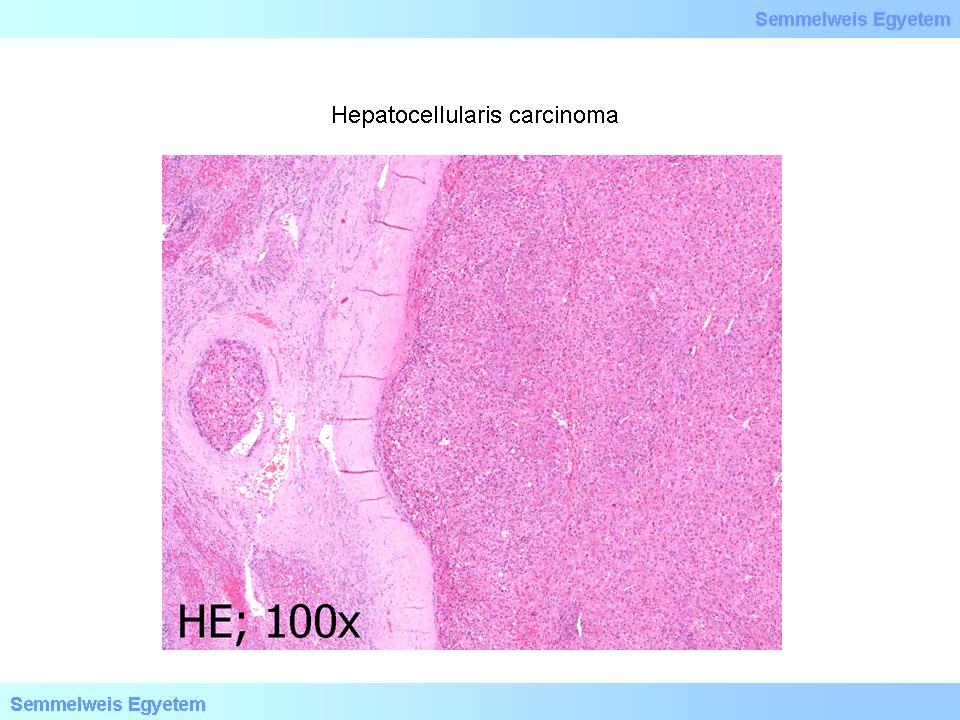
microscopical image No.3 (HE; 100x): Hepatocellular carcinoma. A primary hepatocellular tumor focus is seen which is separated from the surrounding hepatic parenchyma by a pseudocapsule. On the left side of the image there are signs of tumor invasion into a dilated vein. (From the collection of the 2nd Institute for Pathology, Semmelweis University – collected by Ildikó Szirtes).
|
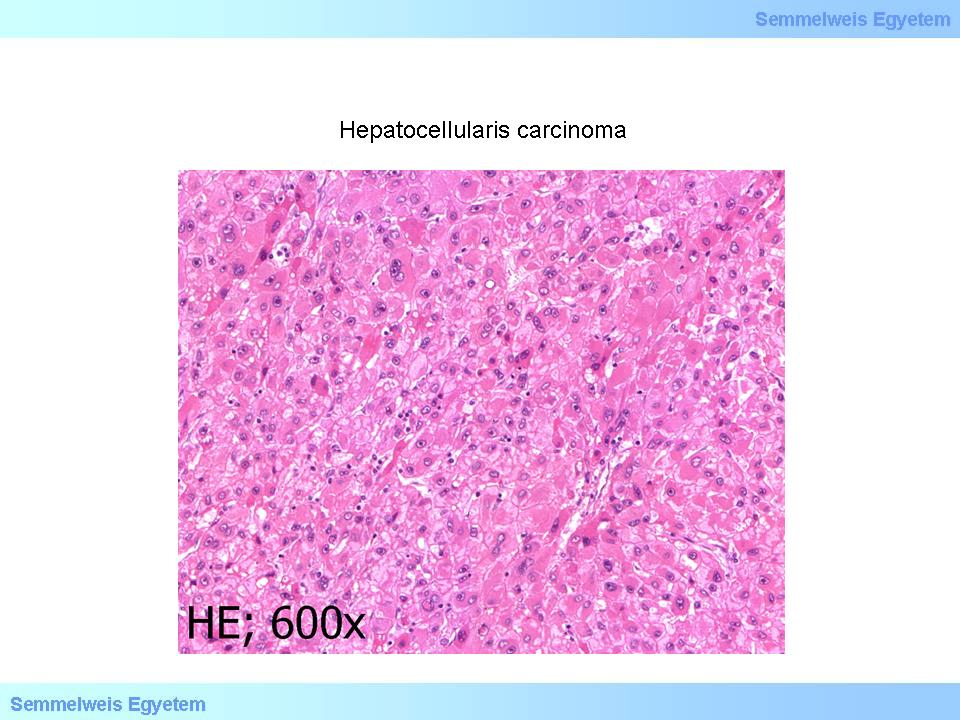
microscopical image No.4 (HE; 600x): Hepatocellular carcinoma. The tumor tissue seen on microscopical image 6P-3 – examined with a higher magnification – consists of moderately polymorphic tumor cells resembling hepatocytes with enlarged nucleus with prominent nucleolus. Tumor type characteristic nuclear inclusions can be detected in severeal nuclei. (From the collection of the 2nd Institute for Pathology, Semmelweis University – collected by Ildikó Szirtes).
|
A special histological form of HCC is fibrolamellar carcinoma. Histologically the tumor cells are large, show vivid eosinophilia, resemble hepatocytes and show trabecular organization. Between the trabeculae there is connective tissue stroma which is rich in fibers and shows bundle structure. It mainly affects young people, and occurs primarily in women. There is no cirrhosis in the tumor free parenchyma. Tumor cells are often positive to some endocrine markers. Its prognosis is better than that of HCCs of other origins.
Another specific form of HCC is the so-called ’minute’ (small) carcinoma which – in accordance with its name – is small, doesn’t exceed 5 cm (or 3 or 3.5 cm according to some authors) by definition. This small tumor has a capsule, and develops on the grounds of a cirrhotic liver.
HCC spreading within the liver – should it happen – is characterized by intrusion into the portal veins and spreading along the biliary ducts. By invasion into the central veins, the tumor metastasize to tissues out of the liver, mainly to the lungs. In case of portal congestion due to baseline hepatic cirrhosis the umbilical vein may reopen and become dilated which may lead to abdominal wall and navel metastases by means of the portocaval anastomosis system known as caput medusae.
Regional lymph node metastases may occur by means of the lymphatic system, mainly in the porta hepatis, and if they grow they may exert pressure on the portal structures thus leading to aggravation of already existing obliteration of biliary flow or portal vein circulation. The tumor may affect the diaphragm too by direct (per continuitatem) spread.
I/2.4.5: Cholangiocellular carcinoma (CCC)
|
 |
A malignant tumor originating from biliary duct epithelium. Those that develop centrally, i.e. close to the porta, are usually larger and unilocular; the so-called Klatskin tumor is a topographically distinguished form which locates at the furcation of the common hepatic duct. Peripherally located tumors are smaller and often multilocular, and they usually develop in cirrhotic liver. Its causes include alcoholism, smoking, Fasciola hepatica infection, and primary sclerosing cholangitis (PSC).
It needs to be distinguished primarily from metastatic adenocarcinomas, especially pancratic and stomach cancer metastases which is often barely possible since the fine structural (histopathological) and immunoreactivity charecteristics are very similar – pancreatic cancer metastases and bile duct cancers are especially alike. This can be explained by the fact that both structures have the same developmental origin, and both of them belong to the so-called ’pancreatobiliary unit’. Primary cholangiocellular carcinomas of the liver are characterized by rapid progression and high recurrence rate, therefore their prognosis is poor.
Macromorphologically it is a greyish-white, compact tumor with blurred outline and no capsule, and sometimes it spreads within the hepatic parenchyma using the portal areas’ system, thus creating a tumor network which is difficult to recognize for the first sight. Histologically it is a regular adenocarcinoma embedded in a compact, fibre-rich neostroma (scirrhus). The glandular or duct structures are of various differentiation, and often contain mucin and/or bile.
Combination tumors (HCC + CCC) occur rarely.
I/2.4.6: Hepatoblastoma
It is a primitive hepatocellular tumor occcurring in childhood. It grows and progresses rapidly, and its prognosis is poor. Macroscopically it is characterised by marrowy tumor tissue surrounded by a pseudocapsule, and the surrounding tumor free hepatic parenchyma is not cirrhotic. It histological subtypes are:
-
- epithelial,
-
- mixed epithelial and mesenchymal.
Serologically, elevated AFP levels are detected. This tumor may be accompanied by developmental disorders of other organs (heart, kidney).
I/2.4.7: Hemangioma
It is a benign solitary or multiple tumor which is usually less than 5 cm in size but larger tumors also occur. Macroscopically it consists of deep red, partially thombotized vessels of various size filled with blood and separated by thin septums. At the sites of previous thrombosis with subsequent organization/scarring, the tumor is whitish and its touch is elastic-compact. Histologically the vessels are lined with flat, typical endothel with peaceful histopathological appearance. Before the introduction of ultrasound diagnostics it was usually a chance finding during autopsy, but today it is detectable by ultrasound during the patient’s life. The risks of subcapsular hemangiomas include spontaneous or trauma associated rupture, subsequent abdominal bleeding and the development of acute abdominal syndrome therefore surgical removal of these tumors which are close to the surface is necessary. A rare, specific form is sclerosing hemangioma.
I/2.4.8: Hemangiosarcoma/hemangioendothelioma
Rare malignant hepatic tumors, and their causes include vinyl-chloride and Thorotrast exposition. Multiple tumor foci appear, and the tumors are characterized by rapid progression and poor prognosis.
I/2.4.9: Secondary (metastatic) hepatic tumors
These are the most common hepatic tumors. The liver has a central location for most organs, therefore it is not surprising that metastatic tumors get to it from several organs. First of all the malignant tumors of the splanchnic area metastasize to the liver (microscopic image No.5), primarily the stomach, pancreas, cholecyst, extrahepatic bile ducts, Vater papilla, small intestines, and large intestines up to the upper third of the rectum (tumors in the lower two-thirds of the rectum metastasize into the lungs through tha caval system!).
|

Study the image!
|

Microscopic image No.5: (HE; 400x) Hepatic metastasis. The hepatic parenchyma is infiltrated by tumor tissue consisting of glandular structures in the metastatic focus. (From the collection of the 2nd Institute for Pathology, Semmelweis University – collected by Ildikó Szirtes).
|
Malignant tumor metastases other than those of the splanchnic area originate primarily from the breast and lungs and we need to count on malignant melanoma metastases from any locations. Macroscopically usually multiple, rarely solitary, delineated, round, non-capsulated, greyish-white tumor foci are seen (macroscopic image No.5) which may show colliquation nectrosis in the middle. The tumor focus which is colliquated in the middle shows a retraction on the surface. Rapidly growing metastases (that can reach even several kilograms) cause enlarged liver and hepatic failure due to space occupation. Multiple metastases show multiple round shadows during imaging examinations (e.g. ultrasound), and this is called lachée des ballons (balloon flight).
|

Study the image!
|
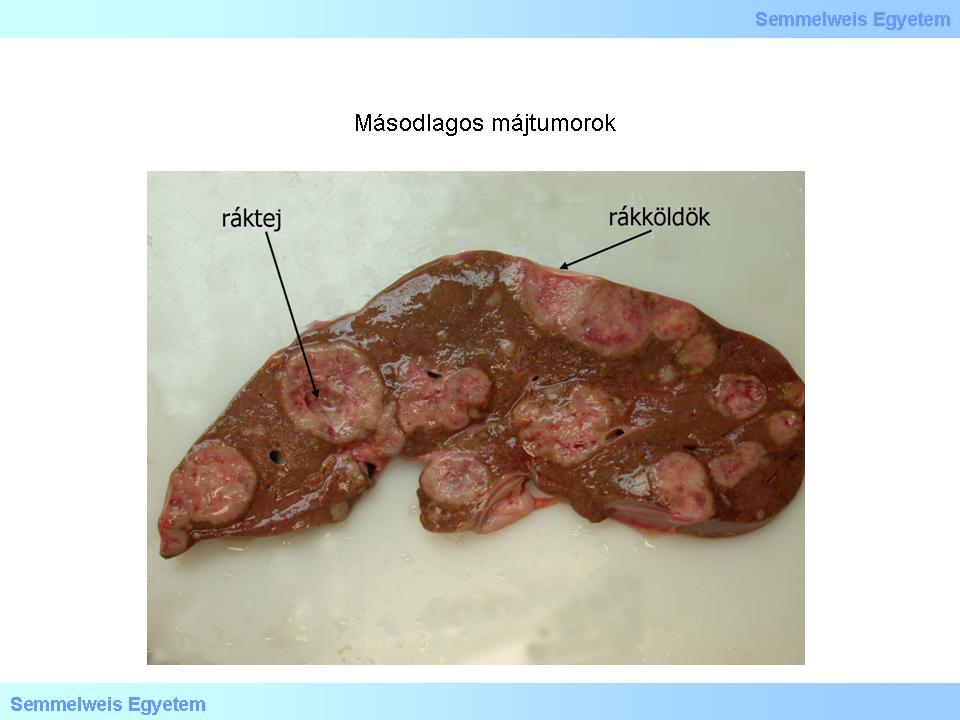
Macroscopic image No.5: Secondary liver tumors. Metastatic foci are almost always multiple, well delineated and usually greyish-white. (From the collection of the 2nd Institute for Pathology, Semmelweis University – collected by Attila Kovács and Ildikó Szirtes).
|
I/2.4.10: Tumor-like lesions
1: Nodular regenerative hyperplasia (NRH)
|
 |
According to its definition it is the formation of non-neoplastic (regenerative) nodules in the liver surrounded by connective tissue capsule. The essence of the process is a local parenchymal atrophy in the liver of any origin followed by compensatory regeneration. It may be caused by portal vein branch thrombosis, hepatic artery branch arteriosclerosis due to ageing, or initial non-cirrhotic lesions of primary biliary cirrhosis.
Nodular regenerative hyperplasia may occur as a sidekick phenomenon in primary or metastatic hepatic tumors or portal hypertension. Histologically the nodules are surrounded by scarified capsule and consist of more than one cell line of regenerative hepatocyte laminae, while the cells of the parenchyma out of the capsule are squeezed and atrophized. In the middle of the nodule is usually a portal area. Among others it is distinguished from cirrhosis by the fact that neighbouring nodules are not separated by connective tissue septum.
2: Partial nodular transformation (PNT)
It is a very rare lesion similar or identical with nodular regenerative hyperplasia, but the nodules are bigger here. The causative role of portal thrombosis is also suggested here but in PNT the larger branches close to the porta are thought to be obstructed.
3: Focal nodular hyperplasia (FNH)
The origin of the lesion is not completely clarified, but according to general suggestion it is a cirrhosis-like hyperplastic-regenerative hepatic tissue response. FNH is usually a solitary nodule but in 20 percent of cases it has a multifocal appearance. The causative role of oral contraceptives is less obvious here than in case of hepatocellular adenomas. In adulthood the incidence is significantly higher in women, the ratio is 2:1 – 8:1 for women. In men its development is primarily associated with chronic alcoholism.
Macroscopically, FNH usually occurs as a subcapsular, compact space occupation, and on the cut surface it includes a central fibrotic area that stretches out star-like processes into the tissue of the nodule. Histologically, all components of the original hepatic lobule can be found. The star-like connective tissue processes parcel the hepatic tissue of the nodule like septa, and this resembles cirrhosis. Bile duct proliferation develops along the septa.
4: Von Meyenburg complex (bile duct hamartoma)
Macroscopically it is a solitary or multiple small whitish nodule, mostly in a subcapsular localization, or sparsely in the liver parenchyma. It is a benign lesion, however, it may deceptively mimic a liver metastasis during surgery which may necessitate intraoperative frozen section histology. Microscopically disordered small bile ducts are seen in a collagen-rich environment.
I/2.4.11: The molecular pathological background of liver cancer (hepatocellular carcinoma)
Our knowledge on the molecular background of the pathogenesis and progression of hepatocellular carcinoma is limited. According to our present knowledge, liver cancer is usually aneuploid; chromosome-gain is verified mainly on 1q, 6p, 8q, 11q, 17q and the whole of the chromosome 7, while chromosome-loss is verified on 4q, 6q, 8p, 13q, 17p and both arms of chromosome 16. Of all chromosomal changes, 7q21-22 region appears to be most linked to metastatic ability, and in search for a related gene in vitro the motility role of PFTK1 (PFTAIRE protein-kinase 1, CDC2-related gene) was verified.
In addition the disturbance of four signal pathways was observed in hepatocellular carcinomában:
|
 |
-
1) pRB pathway,
-
2) WNT pathway,
-
3) STAT pathway, and
-
4) p53 pathway.
-
- In the pRB pathway which influences the cell cycle, disturbances include RB loss or mutation, CDKN2A loss, hypermethylation or mutation, and amplification of cyclin-D1 gene (CCND1) and MYC.
-
- In the WNT pathway CTNNB1 mutation is very common in hepatocellular carcinoma (25-30%) while APC inactivity is rare (thought it is common in colon cancer). Further disorders include axin 1 mutation and decreased e-cadherin activity. The reason is also mutation or promoter hypermethylation here, and the consequence is decreased adherence between the cells, and elevated β-catenin concentration in the nucleus.
-
- STAT pathway activation is caused by promoter hypermethylation of the SOCS1 gene which leads to cessation of JAK2 inhibition, and such target genes as IL6 get activated (IL6 is known as a ’cytokin’which is mostly connected to elements of the immune system, but it can also regulate epithelial cells, mainly hepatocytes). TP53 function is insufficient in most heptocellular carcinomas. In European, American and Japanese hepatocellular carcinomas the mutation affects the whole of the DNA-binding domain, while in case of African or Asian origin the „hot spot” is in codon 249 (AGT instead of AGG, that is serine instead of arginine). This is the mutation which can be artificially induced by aflatoxin B1, and develops maily in case of chronic HBV infection. It was observed that TP53 mutations and WNT pathway disorders often go together.
|
 |
Of the growth factors and receptors the important ones in hepatocellular carcinoma are those which are also important in normal liver. TGF-alpha/EGFR: TGF-alpha may be produced by tumor cells and if they also express EGFR then it is considered as autocrine stimulation. HGF/MET: Unlike TGF-alpha, HGF (hepatocyte growth factor) isn’t produced by epithelial cells, but mesenchymal cells activate during the development of cirrhosis or hepatic cancer (especially the Ito cells which nestle among hepatocytes and endothelial cells in the spaces of Disse), they transform into myoepithelial cells and produce growth factors, e.g. HGF not only for themselves but for tehir environment (tumor cells and other stroma cells) too. HGF effect is increased by MET overexpression. Both MET and EGFR are located on chromosome 7 which is verified to show ’gain’ in hepatocellular carcinoma.
IGF2 and its receptor: IGF2 is overproduced in hepatocellular carcinoma (in Wilms’ tumor this is the central event). In the background there may be LOI (loss of imprinting) like in Wilms’ tumor but other factors may also influence IGF2 function. IGF2 takes effect through IGFR-I or the splicing variant of the insulin receptor, hIR1, and activates the MAPK and what is even more important, the PI3K pathway. Insulin has a substantially similar effect to that of IGF2 but insulin mainly affects metabolism, and IGF2 is a growth factor. IGFR-II decreases the levels of IGF2 since altough it acts as a receptor and binds IGF2, it leads to elimination instead of stimulation (that’s why IGFR-II is considered as a ’scavenger receptor’).
The p53 induced IGFB3 also becomes an inhibiting factor by binding. We can say that IGF2 function depends on IGFR-I and –II expression levels. IGFR-II gene is located on the 6q27 locus where LOH (loss of heterozygosity) is common in hepatocellular carcinoma. Although IGF2 effects are not clarified in hepatocellular carcinoma, its role is not questionable, and this is enforced by activating point mutations of IGFR-I too.
Chronic inflammation of the liver (which can have several causes: hereditary disease, alcohol, virus infection, etc.) makes an ideal environment for genetic mistake accumulation because cell death and regeneration become constant, and the stromal and extracellular matrix elements activate. Regenerative hepatocyte proliferation is often futile because the structure can’t be restored. During these processes, increased cell-replacement (turnover) increases the risk of the most resistant cells to lag behind in the proliferation competition and thus the risk of tumor formation increases.
Viral hepatitises play a leading role among predisposing causes: the risk of hepatocellular carcinoma development increases 200-fold in chronic HBV infection due to the above processes. In HBV infection insertion mutagenesis takes place, and the target gene for cis-activation may be the telomerase gene, or the genes of proteins that affect calcium homeostasis or MAPK pathway. Hepatocellular carcinomas in HBV infection carry integrated HBV DNA which – using the viral enhancer region – may activate proto-oncogenes (e.g. cyclin genes) or inactivate suppressor genes.
The extraneous virus genome usually makes its own incorporation site unstable, thus creating a new ’fragile site’. As a result of trans-activation the integrated virus DNA produces HBx and preS2/S. The amount of HBx becomes significant, and it doesn’t bind to host cell DNA, but may activate or inactivate several genes (e.g. cyclin-A, p53, CREB, ATF2, DDB1, EGFR, TGFβ1, AP2, IL8, VEGF), and activates transcription factors, increases antiapoptotic gene expression (cell immortalization), enhances proliferation, and promotes p53 degradation. The thus created mutants also alter the function of several regulatory systems (e.g. PKC pathway, NFkB, AP1, TGF-alpha, IGF2).
HCV doesn’t integrate into the host cell genom but it affects several regulators, most often via the ’core’ and NS5A-, NS4A- and NS4B genes, or more precisely, through the proteins of these genes. Reactive oxygen species that form as a result of the infection play an important role and enforce the activity of the survival promoting factors (TNF-alpha, Bcl2, NFkappaB, RAF, β-catenin) and inhibit e.g. p53. Various transcription factors also get activated (e.g. SP1, EGR1).
In case of alcohol the damaging factor is the acetaldehyde metabolite which promotes reactive oxygen species formation, inhibits antioxidants, and DNA repair enzyme activity also decreases. Alcohol and HCV together significantly increase the risk of hepatoccellular carcinoma development.
Aflatoxin-B1 mainly occurs and acts as a carcinogen in hot and humid climate. Its metabolites (AFB1-exo-8,9-epoxide, AFB1-FAPY) take part in DNA adduct formation. P53 mutation (in codon 249) and subsequent inactivation is characteristic, as well as decrease of DNA repair efficiency. WNT and Hedgehog pathway errors and other genetic mistakes (e.g. IIGF-2, RASSF1A, p16) are also thought to play a role.
Obesity and diabetes mellitus along with other metabolic disorders and arterial hypertension are the most common causes of nonalcoholic steatohepatitis and cryptogenic cirrhosis. Besides damage caused by reactive oxygen species, e.g. IGF-1 and -2, the insulin receptor and probably the PARP also play a role in malignant transformation.
Among genetic mistakes not associated with any etiologies KRAS mutation and increased RAF production is rare in hepatocellular carcinoma. RAS inactivation may fail due to decreased function of GTPase activating proteins (i.e. RAS inhibiting proteins, e.g. RASSF1A, RASAL1, DAP21P, NF1), most often due to promoter methylation. In the PI3K pathway AKT production increases and PTEN decreases. Of the cell cycle regulators cyclin-D1 gets amplified while cyclin dependent kinase inhibitors (p16, p21, p27) are inhibited, and of the growth factors TGFβ (82%) gets expressed often along with the related mannose-6-phosphate/IGFR-2 and proangiogenic bFGF, VEGF (60%) and PDGF. Other genes’ functions also decrease due to promoter methylation: e.g. E-cadherin, CASP8, TIMOP, MLH1, MSH2, MGMT, SEMA3b, while the activity of DNA-methyltransferase and p-catenin increases, and the latter accumulates in the nucleus.
Of the microRNAs mainly the miR-122 expresses in the adult liver (its role in hepatocellular carcinogenesis is being studied at present). Its targets, i.e. target mRNAs include CAT1, NMYC and cyclin-G1. The expression of miR-145 and miR-199b often decreases, while miR-224 expression increases in the premalignant dysplastic nodules and it remains this way during HBV associated hepatocarcinogenesis too. 17q21-22 gain seems to be related to hepatocellular carcinoma progression.
Seven genes are suggested to play a role in this region:
- 1) There seems to be a relationship between increased PFTAIRE protein-kinase-1 (PFTKI) expression and the motility of „malignamt hepatocytes”, which may contribute to the metastatic ability;
-
2) ODAG;
-
3) CDK6;
-
4) CAS1;
-
5) PEX1;
-
6) SLC25A;
-
7) PEG10.
|

Study the table and analyze what you see!
|
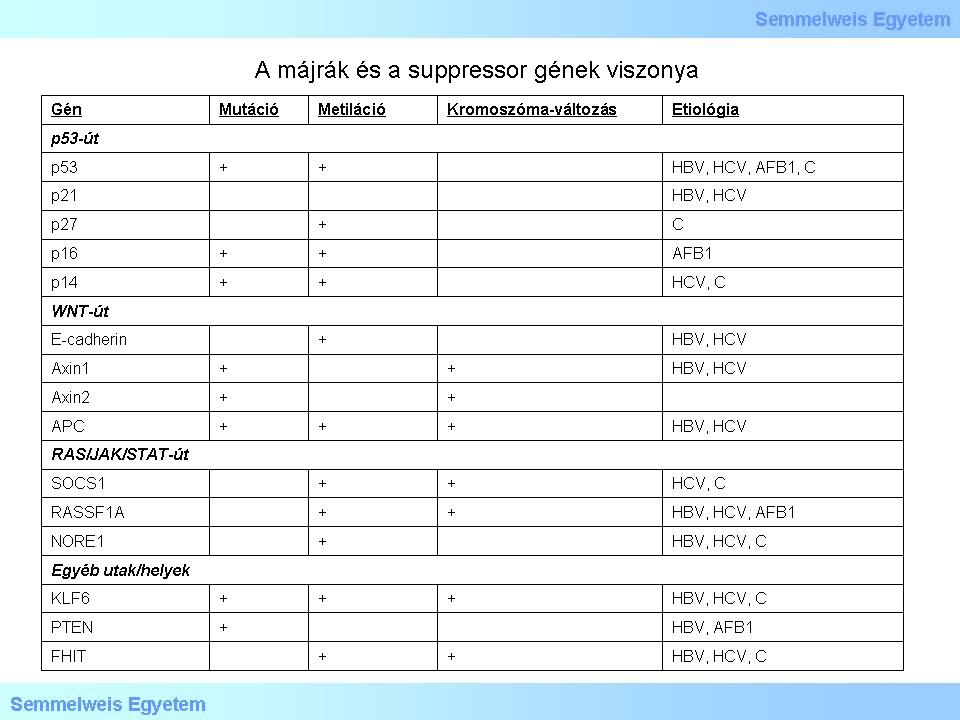
Table 3: The table displays the relationship betweel liver cancer and suppressor genes
|
I/2.4.12: The molecular pathological background of gall bladder and bile duct cancers (cholangiocellular carcinoma)
There is only a few data available regarding gall bladder cancers. P53 mutation which is common in invasive cases and LOH at some chromosome sections (e.g. 8p – 44%, 9p – 50%, 18q – 31%) are considered as early events, although the – supposedly suppressor – genes are not known yet. KRAS mutation (0-34%) is considered as a late event, and HER2 and COX2 amplification were also described.
In case of intrahepatic bile duct cancers KRAS (4-100%, geographical variation is significant) and p53 mutation (cca 30%) are the most common. Increased HER2, MET and Bcl2 expression and decreasing e-cadherin and catenin expression were found. HER2 and COX2 amplification were also described.
KRAS mutation (50-60%) and late p53 mutation are also common in case of extrahepatic bile duct cancers and Vater papilla tumors, and both germinal cell and acquired APC mutation also occur in Vater papilla tumors.
I/2.4.13: Gene silencing (promoter methylation) in hepato- and cholangiocellular carcinoma
|

Study the table and analyze what you see!
|
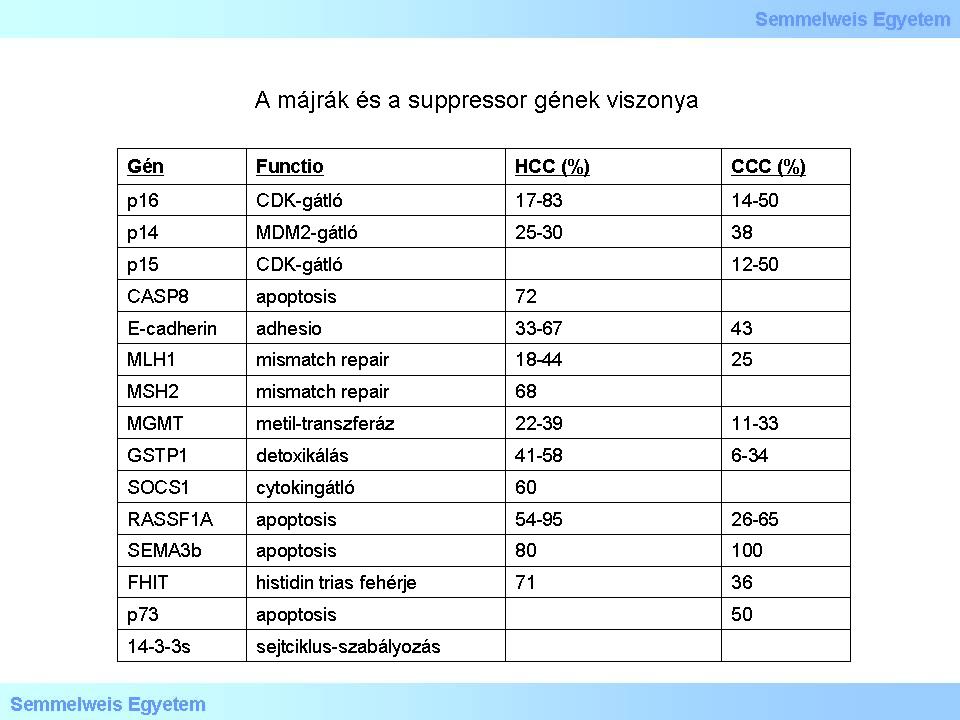
able 4
|
I/2.4.14: Targeted therapy
Conventional chemotherapy brought very modest results in the treatment of hepatocellular carcinoma. This is the reason why therapy against molecular targets was/is received with great expectations. Liver cancer characeristics include RAF and some other tyrosine kinase mutations, therefore it is understandable that sorafenib (multikinase inhibitor, and RAF inhibitor among others) led to significant improvement of survival rate in advanced hepatic cancers as compared with former results. Sorafenib administration is beneficial in the first-line treatment of inoperable hepatocellular carcinoma which is not eligible for local therapy. Clinical studies show promising results with sunitinib which is also a multikinase inhibitor. Combinations such as erlotinib + bevacizumab or sorafenib + doxorubicin are also promising.
In case of increased RAS or RAF activity MEK inhibition in a selected patient group may be a logical therapeutical method (e.g. selumetinib, phase 2). Further possible targets include steps of the PI3K pathway such as mTOR or other signaling pathways, e.g. IGFR inhibitors, and possibly the inhibition of WNT pathway which was unsuccessful so far due to side effects.
|
|