III./3.3.: Postoperative affairs following surgical reconstructions
| |
III./3.3.: Postoperative affairs following surgical reconstructions
Temporary palliation may be necessary in case of DORV, mainly due to the assurance of pulmonary inflow. In the course of designing the reconstructional surgery the two-chamber procedures are preferred obviously. Applied surgical method depends on the situation of great vessels, position of VSD, development of the pulmonary great vessels and accompanying additional congenital heart disorders.
|
 |
Long-term survival is strongly influenced by the fact what kind of reconstruction was possible in the view of accompanying disorders. Important factors are the pressure affairs of lung. If pulmonary hypertension has developed, chances are worser and counterweighting of the patient will be difficult. It is a frequent procedure that the left ventricle’s blood is led to the aorta through the previous VSD, while a new left ventricular outlet is composed. If the reconstruction cannot be carried out using an own pulmonary artery, homograft must be used. However, homografts get old, and replacement may be necessary a decade later. The more practical timing of high-risk surgeries is important in the course of the patient’s life, and their numbers must be kept between reasonable limits. Selection of the right timing is usually decided by a specialized team in possession of invasive and non-invasive check-up data.
More and more patients with reconstructed congenital heart disorders reach the adulthood, and problems, treatment of adult patient group differ from that of the children. Therefore, a separate working group deals with adults in specialized institutions (GUCH – grown-up congenital heart disease). Late problems are determinated by the accompanying developmental disorders and the type of applied surgical method (Figure 3.). Residual VSD can develop on the deflector spots, and the homografts will be calcified. Arterial and atrial switch methods and coarctation have characteristic problems.
|
 |
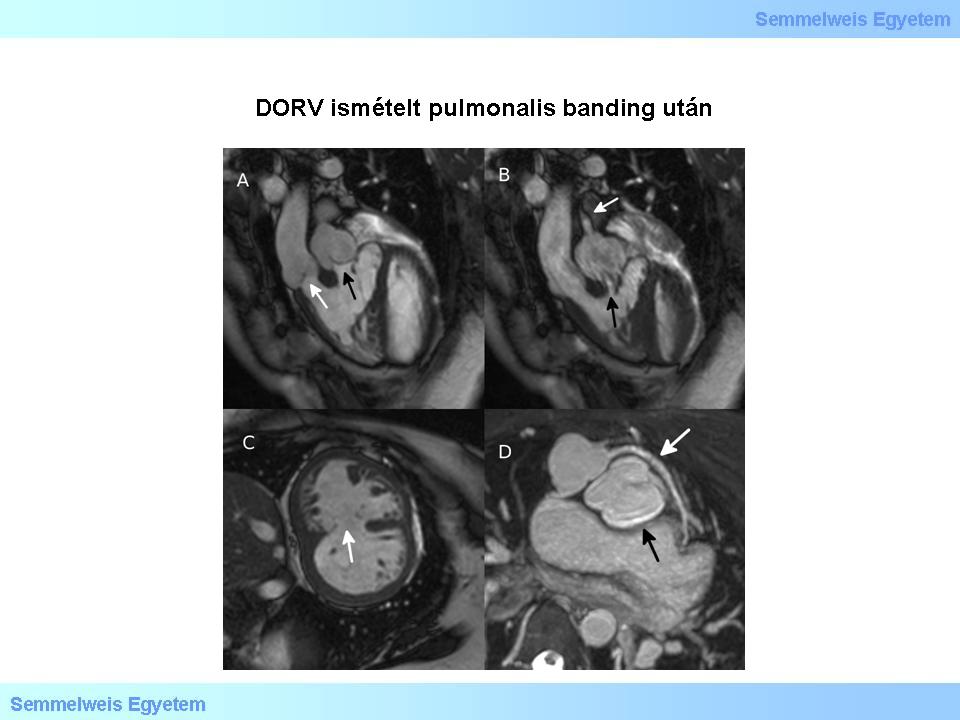
Figure 3.: DORV following repeated pulmonary banding. A: Right ventricular outflow in diastole. White arrow – aortic root; black arrow – pulmonary root. B: Right ventricular outflow in systole. Black arrow – pulmonary stenosis at origin; white arrow – jet indicating pulmonary stenosis following previous banding procedures. C: Hypertrophied right ventricle due to square pressure. White arrow – broad muscular VSD. D: from the transponated aortic root deriving coronary artery with rotational variation. White arrow – coronary artery of variational origin and course, which crosses the pulmonary artery ventrally; black arrow – asymmetric cusps of ectatic pulmonary valve.
|
Peacock published the so-called split right ventricle abnormality first in 1867. The first paper on similar morphology was published by Tsifutis and colleagues in 1961. Muscular and/or fibrotic elements divide the right ventricle. Right ventricular outflow obstruction can also result similar appearence.
|
|
Utolsó módosítás: 2014. May 5., Monday, 10:12