| |
I/2.3: Hepatic cirrhosis
I/2.3.1: Definition
|
| |
Hepatic cirrhosis is a common developmental stage of hepatic diseases of various etiologies, where antagonistic processes of hepatic cell damage (cellular loss, necrosis) and replacemenrt regeneration) are present at the same time, leading to chronic, diffuse, irreversible, so-called pseudolobular remodellation of the hepatic parenchyma. Pseudo-lobules, as the units of remodellation, are typically regenerative and nodular (macro- and/or micronodular); and histologically are characterized by progressive concentric (annular) fibrosis which practically confines the pseudo-lobule from its surroundings and thereby defines its shape.
I/2.3.2: Morphology
I/2.3.2.1: Macroscopic properties
With naked eye, we first see a rather enlarged liver, which becomes shrunken in late stages, and is compact, sometimes even hard, and its outer and cut-surface are uneven with smaller and larger nodules. The colour of the vital parenchyma ranges from yellowish (fatty degeneration) to deep green (cholestasis) or even dark brown (e.g. haemochromatosis), while the scarred parts (fibrosis) are greyish-white.
I/2.3.2.2: Microscopic properties
Histological characteristics are: regenerative nodules in the hepatic parenchyma (pseudo-lobules); concentric fibrotic septa (annular fibrosis) around the nodules; and reactive bile duct proliferation within the fibrosis (microscopic image No.1) with round cell infiltration. Topographically the septa may be porto-portal, porto-central, or centro-central, depending on the etiology. Even though the histological image is not pathognomic, some fine tissue alterations may refer to the possible pathogenesis. For example, severe fatty degeneration of the hepatocytes (microscopic image No.2) or the appearance of so-called Mallory bodies (alcoholic hyaline) are characteristic of alcoholic cirrhosis; viral marker tests are positive in viral cirrhosis; large amount of biliary pigment appears in biliary cirrhosis, copper containing pigment is found when cirrhosis develops on the ground of Wilson’s disease; iron containing pigment is seen in haemochromatosis associated cirrhosis; while specific inclusions are found in some metabolic disorders leading to cirrhosis.
|

Study the images, and analyze what you see!
|

Microscopic image No.1: (HE; 15x) Hepatic cirrhosis. Instead of the usual lobular liver structure, the hepatic parenchyma consists of pseudo-lobules containing chronic inflammatory cells and bile duct proliferation, and surrounded by connective tissue septa (From the collection of the 2nd Institute for Pathology, Semmelweis University – collected by Ildikó Szirtes).
|
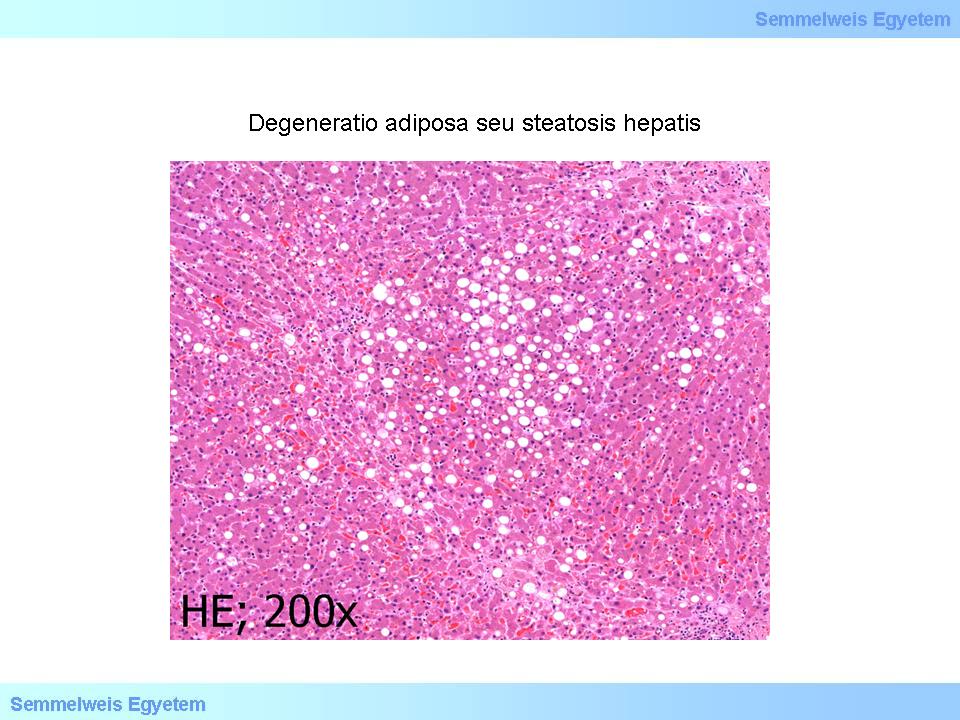
Microscopic image No.2: (HE; 200x) Adipose degeneration or hepatic steatosis. Histopathologic image of large drop fatty remodelling with centrolobular dominance. ’Empty’ vacuoles are seen in the hepatic cell cytoplasms where the fat was dissolved during the histological processing. (From the collection of the 2nd Institute for Pathology, Semmelweis University – collected by Ildikó Szirtes).
|
Connective tissue fibers and extracellular matrix play an important role in the development of cirrhosis. In healthy livers, type I and III collagen fibers are seen in the portal areas and – in a small amont – around the cemtral veins, and type IV collagen is found in the spaces of Disse.
In cirrhotic livers
-
- type I and III collagen fibers form even within the hepatic lobules (during hepatic structure remodellation);
-
- heparan sulphate/chondroitin sulphate ratio inverts in the extracellular matrix;
-
- Ito cells start producing collagen fibers (so-called intralobular fibrosis), therefore the sinusoid walls show fibrotic thickening (this process is erraticly called capillarization)
I/2.3.3: Classification
I/2.3.3.1: Morphological classification
|
 |
In micronodular cirrhosis the size of the nodules doesn’t exceed 3 mm, while in macronodular cirrhosis the nodule size is over 3 mm. If both micro- and macronodules are present, then the cirrhosis is called mixed (macro- and micronodular cirrhosis).
I/2.3.3.2: Etiological classification
|
 |
-
1) Depending on the cause of development cirrhosis may be
-
2) alcoholic,
-
3) viral,
-
4) biliary (primary or secondary),
-
5) toxic, (v) postnecrotic,
-
6) autoimmune,
-
7) metabolic (on the ground of haemochromatosis, Wilson’s disease, alpha-1 antitrypsine deficiency),
-
8) cryptogenic (unclarified origin).
I/2.3.4: Types of cirrhosis
-
1) Alcoholic cirrhosis: The most common cause of cirrhosis in Europe and North America. Morphologically it is typically micronodular cirrhosis (so-called Laennec’s cirrhosis). It is caused by both the toxic effect of alcohol on hepatocytes, and nutritional disorder (lack of protein and vitamine intake).
-
2) Viral cirrhosis: It might develop as a consequence of human hepatotropic virus (HBV, HCV, HDV) infection, as a progression of chronic hepatitis. It is usually micronodular, but in rare cases (e.g. as a consequence of extended necroses caused by fulminant hepatitis) macronodular or mixed cirrhosis may also occur.
-
3) Toxic cirrhosis: It is usually caused by environmental factors, industrial or agricultural substances (contaminants or pollutants): e.g. spraying agents or dissolvents. It may also be a part of an occupational disease. Another typical etiology is food poisoning caused by aflatoxin (via wheat polluted by aflatoxin producing fungi).
-
4) Postnecrotic cirrhosis: This form develops during the regeneration of extended hepatic parenchymal necroses. Its leading causes are: food and mainly mushroom poisoning (uncontrolled amateur mushroom collectors), halothan intoxication, and fulminant viral hepatitis. Extended parenchymal necroses activate the development of bulky regenerative nodules separated by wide and scarred connective tissue septa, which leads to rough deformation of the liver’s outer appearance (so-called potato sack liver).
|
| |
-
5) Biliary cirrhosis: It develops as a result of small or larger bile duct damage with consequent cholestasis which leads to hepatocyte damage, later hepatic tissue necrosis and finally cirrhosis. Therefore the liver’s colour is typically dark green.
Etiologically, bilary cirrhosis can be:
‘primary’ or
’secondary’
- Primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC) are autoimmune diseases of the intrahepatic bile ducts.
- Secondary biliary cirrhosis develops as a result of chronic progressive stenosis of the extrahepatic large bile ducts, due to e.g. intra- or extraluminal neoplastic space occupation, scarred bile duct stricture, stone formation in the bile ducts, or iatrogenic (post-interventional) stricture. Secondary cholestasis is often complicated by ascending cholangitis because this form of cholestasis typically develops over a longer period of time, usually in an intermittent manner, and occlusion is not complete for long, therefore duodenal bacteria have the opportunity to ascend into the static bile, and create microabscesses along the dilated bile ducts.
|
 |
5.1: Primary biliary cirrhosis (PBC): According to its definition, PBC is a chronic progressive non-suppurative cholangitis of the small intrahepatic (interlobular) bile ducts causing scarification and tissue necrosis. PBC leads to progressive scarification and cirrhotic remodellation in the liver parenchyma. Its patomechanism is suggested to be autoimmune. Patients are predominantly women in a 6 to 1 ratio, and most of them are middle aged adults. The leading clinical symptoms are: skin itching and in later stages hyperpigmentation and xanthelasma. Laboratory tests show high serum alkaline phosphatase and serum cholesterol levels, autoantibodies (primarily antimitochondrial antibodies – AMA), and hyperbilirubinemia in later stages.
In the cirrhotic stage the symptoms are similar to those of cirrhosis of any other etiology. PBC is often accompanied by other autoimmune diseases: e.g. Sjögren’s syndrome, rheumatoid arthritis, glomerulonephitises, scleroderma, coeliac disease, or Raynaud’s disease. Histologically 4 stages are distinguished.
-
- In stage I. chronic destructive non-suppurative cholangitis, intense lymphocytic infiltration around the small interlobular bile ducts, and often granuloma formation are seen. Copper-protein complexes often form in the hepatocytes. There is usually no cholestasis in this stage
-
- In stages II and III progressive bile duct proliferation and scarification are seen with cholestasis.
-
- In stage IV classic cirrhosis is seen with significant cholestasis.
5.2: Primary sclerosing cholangitis (PSC) It is an inflammatory fibrosing disease of the medium and large (intra- and extrahepatic) bile ducts leading to progressive stenosis. The radiological picture is typical: irregular narrowed and dilated bile ducts alternate. Autoimmune etiology is suggested. Gender ratio shows male predominance (2 to 1). In 70% (!) of the cases, PSC is accompanied by ulcerative colitis. The leading clinical symptoms are fatigability, itching (pruritus), and increasing jaundice; while laboratory tests show elevated serum alkaline phosphatase level.
PSC may progress into cholangiocellular carcinoma. The only successful treatment option is liver transplant. Histomorphologically a fibrosing progressive cholangitis is seen, where collagen fibers are distributed concentrically around the affected bile ducts in an onion skin pattern with massive lymphocyte infiltration. This leads to bile duct stenosis and occlusion with endothelial damage, dilation of prestenotic bile duct segments (cholangiectasia), and cholestasis. The end-stage is biliary cirrhosis.
|
| |
-
6) Autoimmune cirrhosis: Morphologically it is micronodular cirrhosis, and develops during the progression of autoimmune hepatitis. Serologically autoantibodies are detectable. (Due to their autoimmune etiologies, both PBC and PSC could be classified in this group.)
-
7) Metabolic cirrhosis: Morphologically it is ususally micronodular cirrhosis.
1: Haemochromatosis
Hemosiderin (besides macrophages) is stored by the cells of several large parenchymal organs (heart, liver, kidney). This causes cell damage in the affected organs, and that leads to a colorful clinical picture consisting of simultaneous function disorder and failure symptoms of several organs which doesn’t seem to be connected. Haemochromatosis causes hepatocyte damage, progressive fibrosis and cirrhosis in the liver.
2: Wilson’s disease
The essence of the disease is copper overload induced tissue damage, not only in the liver but in other organs too – like in the brain, cornea, or kidney. It is a rare autosomal recessive disease, the genetic mutation is located on the 13q14-21 locus. Besides cirrhosis, Wilson’s disease may present clinically and histologically as fulminant, acute, or chronic hepatitis. In case of the rare fulminant disease form, which may occur even in the clinical state of perfect well-being and leads to death within a few days, the cause of death is parenchymal hepatic failure, and the pathological picture is acute yellow atrophy of the liver.
In the early stage, steatosis is seen with periportal lipofuscin accumulation. In the stage of progressive fibrosis, septa grow from the portal areas which develop into cirrhosis in untreated patients. Histological signs of diagnositic value are the following: steatosis, hepatocyte ballooning, gylcogen saturation in the nuclei, Mallory bodies in the periportal hepatocytes, copper deposition in the hepatocytes (they can be detected by e.g. rhodanine or silver staining).
3: Alpha-1 antitrypsin deficiency
Alpha-1 antitrypsin is a protease inhibiting serum glycoprotein which is produced upon inflammatory and hormonal stimuli, and in case of hepatic disease it acts as an acute phase controlling enzyme. In alpha-1 antitrypsin deficiency, an amino acid substitution occurs at position 342 (lysin is replaced by glutamine), which leads to damaged spatial structure, the enzyme gets stuck in the rough endoplasmic reticulum, and it becomes unable to secrete enzyme product which leads to deficiency symptoms in the serum.
The most frequent deficient allel variations are PiZ and PiS; and hepatic disorders (cirrhosis, hepatocellular and cholangiocellular primary liver cancer) are primarily associated with PiS.
|
 |
Histologically, periportal hepatocytes contain eosinophilic, diastase-resistant, PAS-positive globular inclusions measuring 1-10 microns, and these inclusions are actually retained alpha-1 antitrypsin products. Tissue damage derives from uncontrolled inflammatory response which occurs upon every infections however mild they are, but due to lack of control mechanism, it lasts long or becomes practically permanent because of the constant biological-microbial load affecting the body, and destroys not only the infective agents but the baseline structure as well. This leads to hepatic tissue damage and later cirrhosis, and this is how patients with alpha-1 antitrypsin deficiency develop emphysema as a result of pulmonary fibre-structure damage.
I/2.3.5: Consequences and complications of cirrhosis
|
| |
Pseudolobular remodelling of the hepatic parenchyma and the annular fibrotic septal system that practically divides the parenchyma to several closed compartments, hinder hepatic function in two main aspects:
-
(i) on the first hand it cuts the original route of blood several times and hinders its flow through the liver (circulatory hepatic failure, hepatic vascular decompensation) with all the consequences,
-
(ii) and on the other hand it separates hepatic cells from their environment, thus preventing them to perform their parenchymal tasks (e.g. secrete their products or uptake substances that should be broken down/detoxificated – parenchymal hepatic failure, parenchymal hepatic decompensation)
1: Signs of vascular hepatic failure
Portal hypertension means congestion of the whole splanchnic area. Esophagal varices – with or without rupture – are caused by the dilation of the most important portocaval anastomosis due to redistribution of the blood flow diversion. Their rupture leads to often life threatening gastrointestinal bleeding (it is the direct cause of death in 30-40% of all cirrhosis cases). Caput medusae or paraumbilical venous dilation is also a form of portocaval dilation, as well as perianal venous dilation (hemorrhoids) and dilation of the retroperitoneal vascular system.
Ascites (abdominal dropsy) develops as a result of trasperitoneal diffusion due to increased splanchnic intravasculary pressure: 500 mL to several litres of transudate accumulates in the abdominal cavity. In case of spleen enlargement (fibro-congestive splenomegaly) the spleen may weight several hundred grams, and it becomes compact. In the background of so-called diffusion or spontaneous peritonitis there is portal hypertension that leads to chronic bowel wall hypoxia and results in increased permeability, enteral bacterial diffusion, and eventually peritonitis without perforation.
2: Signs of parenchymal hepatic failure
Jaundice (icterus) is the result of mostly conjugated and sometimes unconjugated hyperbilirubinemia. Hypoalbuminemia is caused by hindered protein production and leads to generalized edema. Hemophilia is caused by hepatic coagulation factor deficiency (fibrinogen, prothrombin, factor V, VII, IX, X). Decreased hepatic estrogen hormon metabolism leads to hyperestrogenism, which manifests in feminisation of male patients: testicular atrophy (hypogonadism), impotence, gynecomastia, loss of masculine body hair (e.g. hairless abdomen). Spider naevi are small angiomas on the skin that develop on the ground of hyperestrogenism.
Hepatic encephalopathy may range from sleep-wake cycle disorders (stage 1) to hepatic coma (stage 4). It is caused by obstructed elimination (detoxication) of potentially neurotoxic substances containing nitrogen (NH3, γ-aminobutyric acid/GABA). Central nervous system symptoms (consciousness disturbance) become even worse during episodes of bleeding from esophagal varices, because blood supply disorders add to metabolic brain tissue damage. Foetor hepaticus is a sweetish stink of the exhaled air caused by mercaptans, which are produced by enteral bacteria during methionin metabolism. The risk of malignant degeneration – transition into hepatocellular carcinoma (HCC) – is increased by constant regenerative cell division and subsequently the probability of error becomes statistically significant. Plantar and palmar erythema are caused by local vasodilation.
I/2.3.6: Numerical scoring of hepatic cirrhosis progression (i): grading based on HAI – Histological Activity Index (so-called necroinflammatory evaluation)
|

Study the tables!
|
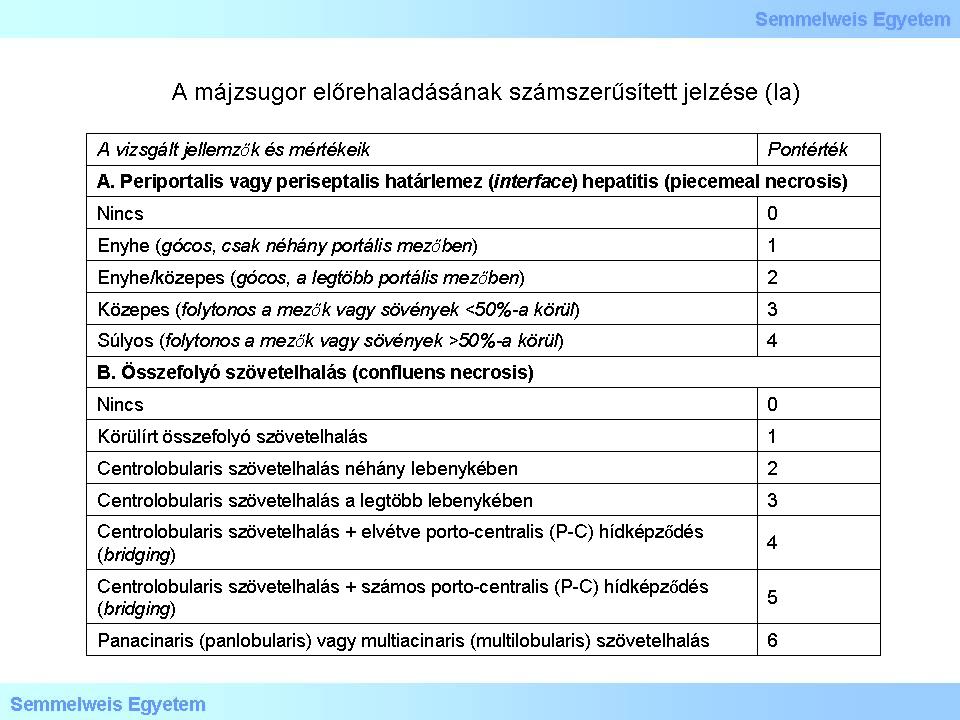
Table 1a: Numerical scoring of hepatic cirrhosis progression (Ia)
|

Table 1b: Numerical scoring of hepatic cirrhosis progression (Ib)
|
The maximal score for grading based on necroinflammatory evaluation is 18. Further parameters to be evaluated but shall not be scored are:
-
1) biliary duct inflammation and damage,
-
2) presence of lymphatic follicles,
-
3) degree of steatosis (none, mild, moderate, severe),
-
4) hepatocellular dysplasia (large or small cell),
-
5) adenomatous hyperplasia,
-
6) iron or copper overload,
-
7) cell inclusions (e.g. PAS-positive intracellular globules, Mallory bodies), and
-
8) immunohistochemical data, such as e.g. detection of viral antigens or lymphocyte subgroups
I/2.3.7: Numerical scoring of hepatic cirrhosis progression (ii): staging based on HAI – Histological Activity Index (so-called fibrosis evaluation)
|

Study the tables!
|
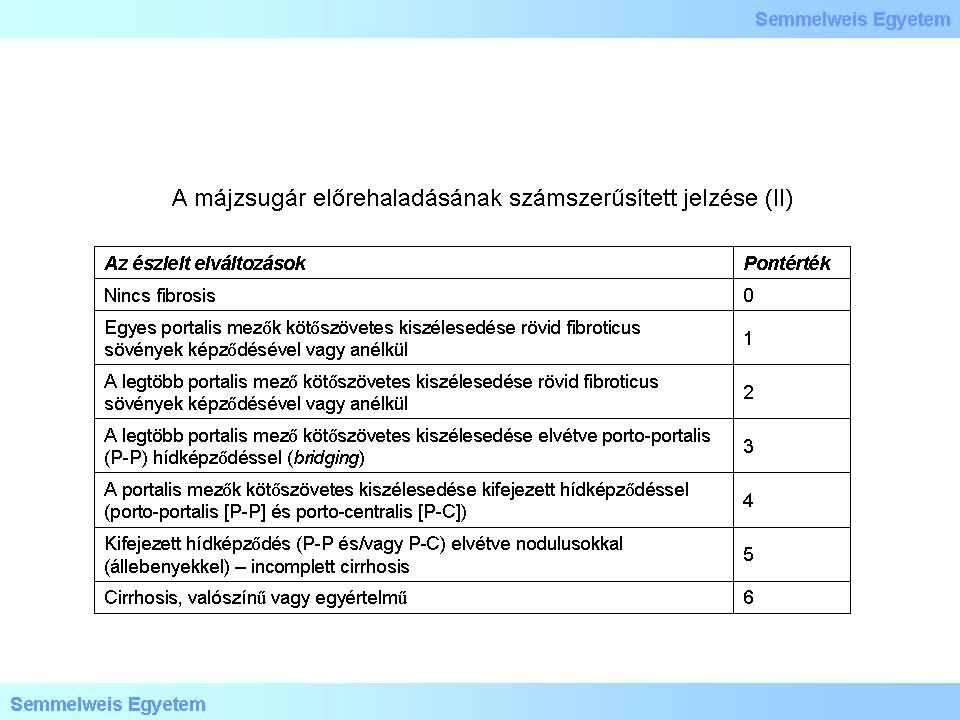
Table 2: Numerical scoring of hepatic cirrhosis progression (II)
|
The maximal score for staging based on fibrosis evaluation is 6. Further characteristics to be evaluated but shall not be scored are:
-
1) acinar fibrosis,
-
2) perivenular (chicken-wire) fibrosis,
-
3) phlebosclerosis of terminal hepatic venulae.
|
|